|
 |
- Search
| J Neurointensive Care > Volume 6(2); 2023 > Article |
|
Abstract
Background
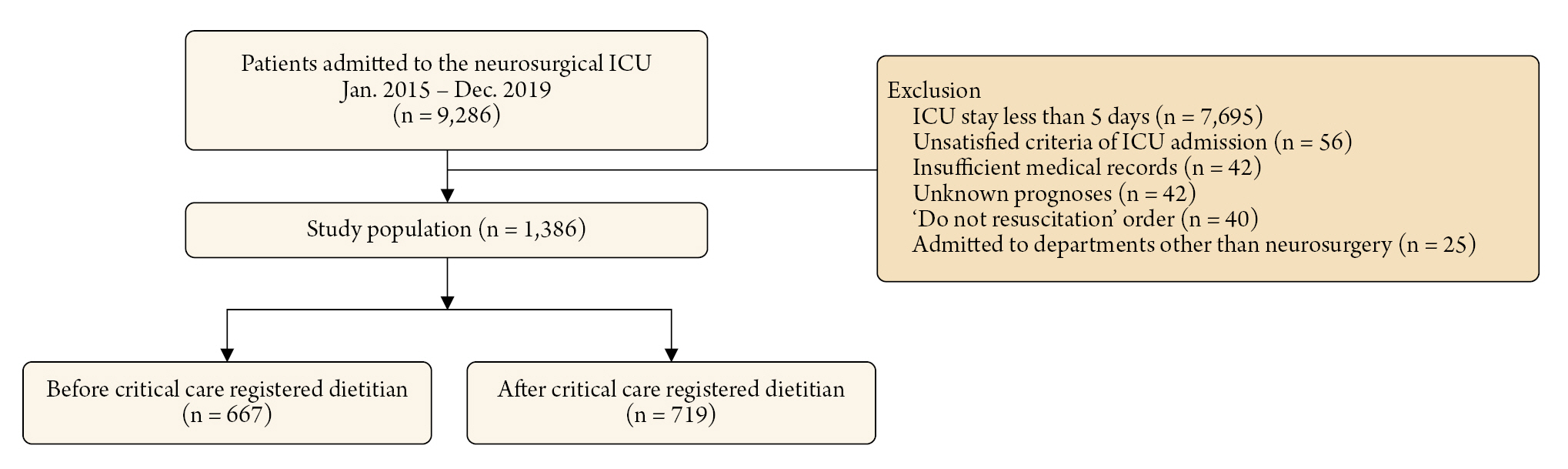
Methods
Results
NOTES
Ethics statement
The study was approved by the Institutional Review Board (IRB) of Samsung Medical Center (No. SMC 2020-09-082) and patients' records were reviewed and published in accordance with the Declaration of Helsinki. Due to the retrospective nature of the study, the requirement for informed consent was waived by the IRB.
Author contributions
Conceptualization: HK, HJK, JAR. Methodology: HJK, JAR. Data curation: HK, JAR, Writing – original draft: HK, JAR. Formal analysis: All authors.
Acknowledgements
We would like to express our gratitude to Suk Kyung Choo, the nursing director of the neurosurgical intensive care unit, for her valuable advice and insightful discussions. Additionally, we extend our thanks to all the nurses in the neurosurgical intensive care unit at Samsung Medical Center for their exceptional work.
Table 1.
|
Overall study population |
||||
|---|---|---|---|---|
| Before (n = 667) | After (n = 719) | p value | SMD | |
| Patient demographics | ||||
| Age (year) | 49.2 ± 23.82 | 49.1 ± 24.0 | 0.964 | 0.002 |
| Sex, male | 322 (48.3) | 380 (52.9) | 0.099 | 0.092 |
| Comorbidities | ||||
| Malignancy | 396 (59.4) | 449 (62.4) | 0.263 | 0.063 |
| Hypertension | 250 (37.5) | 231 (32.1) | 0.042 | 0.113 |
| Diabetes mellitus | 95 (14.2) | 102 (14.2) | 0.999 | 0.002 |
| Chronic kidney disease | 38 (5.7) | 50 (7.0) | 0.396 | 0.052 |
| Cardiovascular disease | 24 (3.6) | 23 (3.2) | 0.793 | 0.022 |
| Chronic liver disease | 23 (3.4) | 19 (2.6) | 0.473 | 0.047 |
| Behavioral risk factors | ||||
| Current alcohol consumption | 119 (17.8) | 154 (21.4) | 0.108 | 0.09 |
| Current smoking | 60 (9.0) | 74 (10.3) | 0.468 | 0.044 |
| Cause of ICU admission | 0.028 | 0.224 | ||
| Brain tumor | 292 (43.8) | 326 (45.3) | ||
| Intracerebral hemorrhage | 86 (12.9) | 125 (17.4) | ||
| Traumatic brain injury | 82 (12.3) | 65 (9.0) | ||
| Subarachnoid hemorrhage | 81 (12.1) | 82 (11.4) | ||
| Elective vascular surgery | 58 (8.7) | 65 (9.0) | ||
| Spinal surgery | 18 (2.7) | 16 (2.2) | ||
| Central nervous system infection | 14 (2.1) | 16 (2.2) | ||
| Cerebral infarction | 9 (1.3) | 13 (1.8) | ||
| Others | 27 (4.0) | 11 (1.5) | ||
| APACHE II score on ICU admission | 6.6 ± 6.4 | 5.9 ± 5.8 | 0.048 | 0.106 |
| Glasgow coma scale on ICU admission | 13.2 ± 3.5 | 13.5 ± 3.1 | 0.084 | 0.093 |
| ICU management | ||||
| Mechanical ventilation | 357 (53.5) | 382 (53.1) | 0.926 | 0.008 |
| Continuous renal replacement therapy | 22 (3.3) | 9 (1.3) | 0.017 | 0.138 |
| ICP monitoring | 279 (41.8) | 279 (38.8) | 0.275 | 0.062 |
| Use of mannitol* | 290 (43.5) | 336 (46.7) | 0.245 | 0.065 |
| Use of glycerin* | 200 (30.0) | 289 (40.2) | <0.001 | 0.215 |
| Use of vasopressors | 52 (7.8) | 178 (24.8) | <0.001 | 0.472 |
| Early nutrition† | 157 (23.5) | 202 (28.1) | 0.068 | |
| Early enteral nutrition | 90 (13.5) | 79 (11.0) | 0.179 | |
| Early parenteral nutrition | 67 (10.0) | 123 (17.1) | <0.001 | |
| Clinical outcomes† | ||||
| In-hospital mortality | 121 (18.1) | 127 (17.7) | 0.872 | |
| 28-day mortality | 108 (16.2) | 125 (17.4) | 0.602 | |
| ICU mortality | 93 (13.9) | 106 (14.7) | 0.728 | |
| ICU length of stay (hour) | 257.2 ± 768.7 | 198.4 ± 206.5 | 0.049 | |
| Hospital length of stay (day) | 80.1 ± 284.0 | 45.5 ± 134.4 | 0.003 | |
Table 2.
|
PSM adjusted population |
||||
|---|---|---|---|---|
| Before (n = 542) | After (n = 542) | p value | SMD | |
| Patient demographics | ||||
| Age (year) | 46.4 ± 24.3 | 47.6 ± 25.0 | 0.423 | 0.049 |
| Sex, male | 274 (50.6) | 268 (49.4) | 0.761 | 0.022 |
| Comorbidities | ||||
| Malignancy | 353 (65.1) | 342 (63.1) | 0.527 | 0.042 |
| Hypertension | 177 (32.7) | 184 (33.9) | 0.699 | 0.027 |
| Diabetes mellitus | 70 (12.9) | 78 (14.4) | 0.536 | 0.043 |
| Chronic kidney disease | 28 (5.2) | 29 (5.4) | 0.999 | 0.008 |
| Cardiovascular disease | 14 (2.6) | 16 (3.0) | 0.853 | 0.022 |
| Chronic liver disease | 14 (2.6) | 17 (3.1) | 0.716 | 0.033 |
| Behavioral risk factors | ||||
| Current alcohol consumption | 105 (19.4) | 107 (19.7) | 0.939 | 0.009 |
| Current smoking | 51 (9.4) | 50 (9.2) | 0.999 | 0.006 |
| Cause of ICU admission | 0.996 | 0.067 | ||
| Brain tumor | 273 (50.4) | 261 (48.2) | ||
| Intracerebral hemorrhage | 73 (13.5) | 76 (14.0) | ||
| Traumatic brain injury | 46 (8.5) | 52 (9.6) | ||
| Subarachnoid hemorrhage | 54 (10.0) | 57 (10.5) | ||
| Elective vascular surgery | 53 (9.8) | 51 (9.4) | ||
| Spinal surgery | 13 (2.4) | 15 (2.8) | ||
| Central nervous system infection | 12 (2.2) | 14 (2.6) | ||
| Cerebral infarction | 8 (1.5) | 7 (1.3) | ||
| Others | 10 (1.8) | 9 (1.7) | ||
| APACHE II score on ICU admission | 5.8 ± 5.6 | 6.3 ± 6.1 | 0.158 | 0.086 |
| Glasgow coma scale on ICU admission | 13.6 ± 3.0 | 13.6 ± 2.9 | 0.951 | 0.004 |
| ICU management | ||||
| Mechanical ventilation | 259 (47.8) | 271 (50.0) | 0.504 | 0.044 |
| Continuous renal replacement therapy | 6 (1.1) | 7 (1.3) | 0.999 | 0.017 |
| ICP monitoring | 239 (44.1) | 234 (43.2) | 0.806 | 0.019 |
| Use of mannitol* | 238 (43.9) | 228 (42.1) | 0.581 | 0.037 |
| Use of glycerin* | 182 (33.6) | 183 (33.8) | 0.999 | 0.004 |
| Use of vasopressors | 51 (9.4) | 60 (11.1) | 0.423 | 0.055 |
| Early nutrition† | 135 (24.9) | 162 (29.9) | 0.088 | |
| Early enteral nutrition | 80 (14.8) | 65 (12.0) | 0.212 | |
| Early parenteral nutrition | 55 (10.1) | 97 (17.9) | <0.001 | |
| Clinical outcomes† | ||||
| In-hospital mortality | 76 (14.0) | 79 (14.6) | 0.862 | |
| 28-day mortality | 68 (12.5) | 79 (14.6) | 0.375 | |
| ICU mortality | 56 (10.3) | 63 (11.6) | 0.560 | |
| ICU length of stay (hour) | 264.4 ± 838.6 | 194.7 ± 213.0 | 0.061 | |
| Hospital length of stay (day) | 63.6 ± 154.0 | 42.9 ± 116.82 | 0.013 | |
Table 3.
| Adjusted Odds Ratio (95% CI)a | p value | |
|---|---|---|
| Overall population | ||
| Critical care registered dietitian | 1.23 (0.76 – 2.00) | 0.409 |
| EEN | 0.27 (0.09 – 0.68) | 0.011 |
| EPN | 0.40 (0.18 – 0.82) | 0.018 |
| Propensity score-matched population | ||
| Critical care registered dietitian | 1.35 (0.77 – 2.37) | 0.295 |
| EEN | 0.32 (0.09 – 0.90) | 0.051 |
| EPN | 0.35 (0.12 – 0.86) | 0.034 |
REFERENCES
- TOOLS
-
METRICS

-
- 0 Crossref
- 1,497 View
- 10 Download
- Related articles in JNIC
-
Association of Obesity With Clinical Outcomes in Neurocritically Ill Patients2022 October;5(2)
Association of Hyponatremia and Clinical Prognosis in Neuro Critically Ill Patients2021 April;4(1)
Prognostic Value of Early Hyperglycemia in Neurocritically Ill Patients2020 April;3(1)