 |
 |
- Search
| J Neurointensive Care > Volume 6(2); 2023 > Article |
|
Abstract
Chronic subdural hematoma (CSDH) is a common neurosurgical disease encountered by neurologists, neurosurgeons, intensive care specialists, and emergency physicians in the emergency department. Although much has been published on CSDH, it remains a topic of active research, also a significant challenge in specific scenarios. The spectrum of clinical presentation varies from asymptomatic radiological entity to significant sensory-motor deterioration resulting in a comatose state. The majority of these cases have an underlying history of trivial trauma in one or the other form. More often, elderly individuals present with CSDH. Management of CSDH in elderly individuals presents specific challenges due to pre-existing comorbidities and medications which alter the pathophysiology. There exists a significant diversity in the treatment modality of CSDH amongst neurosurgeons. The treatment modality includes medical management as well as various forms of surgical manoeuvres intended to evacuate the hematoma and hence alleviate the mass effect over the surrounding brain matter. The treatment modality needs to be individualized for every case. The rationale selection of cases for medical and surgical intervention with robust follow-up results in a better prognostication of these cases.
The Chronic Subdural Hematoma (CSDH) is an encapsulated crescentic collection of fluid, blood, and blood degradation products layered between the dura and arachnoid covering the brain surface which is localized between the dural border cell layer occurring 3 weeks or 21 days after a mild to moderate traumatic brain injury episode1,2).
In general, intensive care unit (ICU) management, it is considered that the general condition of the patient is low, i.e., glasgow coma scale (GCS) ≤12. Briefly, management involves assessment for neurological deficits. Assessment of blood investigations like complete blood count, electrolytes, liver function test, and coagulation studies is mandatory if the GCS ≤ 8 endotracheal intubation is warranted3). Reversal of Coagulopathy, if there is one. If the patient has active cerebral herniation temporary use of hyperosmotic or hypertonic agents before surgery will be beneficial to reduce the ICP [Intra cerebral pressure]. Antiepileptic medications are started keeping in mind that a new onset seizure can increase morbidity and mortality of the patient4). The routine use of corticosteroids is generally not recommended as there is no solid evidence.
The incidence of CSDH is not well established, Yang & Huang indicate in their study that its incidence has been increasing in recent years5). According to a 2017 study report, CSDH incidence was estimated between 3.4 to 58 persons per 100,000 person-years (considering that the value might change depending on the population age), the median age of 63 years old the average for suffering from CSDH but the population aged the median age from suffering will too (follow accordingly)2). The prevalence and relation between males and females are 3:12). In the elderly population, the CSDH has been referred to as a sentinel event related to a concomitant systemic pathology and 1-year mortality2,6).
The specific formation of CSDH is not well-established, according to some literature, CSDH might be caused by the chronic usage of anticoagulant and antiplatelet combined especially in the eldest population, and by inflammatory responses, a pressure increment between the hematoma and blood vessels, chronic alcohol consumption due to the associated brain volume reduction), and as previously mentioned, head trauma antecedent 2,6). Also, according to some recent studies, acute SDH (aSDH) leads to multiple physiologic sequelae (angiogenesis, vascular permeability factor release, and growth factor release) that will develop the CSDH7). CSDH might be developed after an acute subdural hematoma (due to the incomplete blood product resorption) or a subdural hygroma and the inflammatory response that leads to neovascularization due to the angiogenic factors of the inflammation process. In both cases, a substantial inflammatory response seeps into the subdural space. This inflammation includes angiogenic factors that lead to the formation of new blood vessels. Although these fragile capillaries are forming, they experience microbleeds, which can lead to SDH. Enclosed chronic subdural haemorrhage cavity prevents clearance of fibrinolytic enzymes inflammatory cytokines and angiogenic factors. This initiates a cascade of inflammation, fibrinolysis, angiogenesis, and rebleeding (Fig. 1)2).
First proposed by Inglis in 19478). These are layers of flattened elongated cells connected by desmosomes with extracellular matrix and extracellular fibres. Chronic subdural haemorrhage is formed between dural border cells which is confirmed by electron microscopic studies9). The new membrane formation is due to inflammation and pro collagens type one and type 3 collagens. Inflammation causes pro-angiogenic cells which produce new leaky blood vessels which cause microhemorrhages and fluid exudates into the newly formed sub-dural membrane (Fig. 2).
Chronic subdural haemorrhage is more hyperosmolar than cerebrospinal fluid [due to increased protein content by liquefaction of hematoma]10). However, this theory was disproved by Weir, who demonstrated that the osmolality of the hematoma fluid was identical to that of blood and cerebrospinal fluid11). This concept was further substantiated by Taguchi et al. in their study of the resorption of CSDH fluid after surgery. In that study, the authors found that the attenuation rates of radioactivity (due to 111-In-DTPA installation in subdural space) were faster after the surgery12). As the osmotic pressure is the same for the CSDH hematoma fluid, blood, and cerebrospinal fluid (CSF), the osmotic pressure difference alone cannot explain the faster attenuation rate after surgery. The hematoma gets absorbed in the sinusoidal channel layer and therefore the colloid osmotic pressure explains the phenomena better.
In the place where Bridging veins travel from the cortex into the subarachnoid space, it is thicker. Just before its entrance to the Dural border cells and inside the Dural border cells these veins become very thin. And inside the dural border cell layer, these veins have a single layer of endothelium and a single layer of collagen, and no surrounding arachnoid trabeculae. This is the place where the tearing of veins happens13).
First reported by Virchow in 1857 as “pachymeningitis hemorrhagic interna”
a- cytokines induced by inflammation (interleukin- 1,6,8,10)14)
b- chemokines
The membrane of CSDH is composed of an outer and inner layer. The outer layer of the membrane which is toward the inner side of the dura is composed of vascularized granulation tissue15). This layer can be 1 cm thick and is found to be composed of inflammatory tissue consisting of fibroblasts, collagen, and endothelial cells with fenestration and gap junctions16).In contrast to this, the inner membrane is relatively avascular and is more like the arachnoid membrane17). Hong et al. found that inflammation plays a role in the propagation of CSDH based on their findings of increased interleukin-6, vascular endothelial growth factor, and basic fibroblast growth factor in the recurrence of CSDH18).
Elderly, male sex, epilepsy, decreased intracranial pressure states, hemodialysis, chronic alcohol consumption (or abuse), therapeutical interventions (e.g. ventricular shunting, lumbar puncture, spinal anaesthesia, spinal surgery with dural tear and CSF leak), falls and trivial head trauma (especially in the eldest population), and anticoagulant and antiplatelet usage7). Diseases related to brain atrophy- Alzheimer’s, systemic diseases like liver and kidney diseases.
The CSDH can present stroke or progressive dementia signs that can confuse the diagnosis19), due to this unspecific clinical presentation is known as “the great imitator”, as well, its symptoms can onset many weeks before its presentation2). Patients might present seizures, memory disturbances, headaches, speech and gait disturbances, cognitive decline, confusion, hemiparesis, falls and altered mental status that can range from acute confusion deteriorating to even coma1).
The computed Tomography Scan (CT-Scan) is the main imaging modality for CSDH diagnosis; however, Magnetic Resonance Imaging (MRI) is also useful but not preferred1). On the CT Scan, the CSDH appears as a crescent-shaped hypodense collection distributed by the convexity of the brain (Table 1)2). The diagnosis of chronic subdural hematoma is made through neuroimaging, the study of choice is non-contrast computed tomography of the skull1), given its high availability and non-invasive nature. The characteristic of the image obtained in this pathology is a crescent formation due to the collection of blood products in the subdural space, between the arachnoid and the dura mater; Radiodensity measured in Hounsfield units depends on the time of evolution of the lesion due to hemosiderin degradation. It is known that the density decreases by approximately 1.5 units per day20), therefore, in CSH, it is expected to find a hypodense lesion, without ruling out being able to find isodense or mixed lesions (acute-on-chronic); Through this type of image, it is possible to assess the size, thickness, presence of subdural clots, their extension through the sutures (unlike epidural), if it generates a mass effect deviating the midline21), the presence of locations and/or membranes within the hematoma22).
1. Homogeneous: Collections appear homogeneously isodense, hypodense, or hyperdense. Here the Risk of enlargement of hematoma and recurrence is 10 to 15% (Fig. 3)23,24)
2. Laminar [mixed]- A thin high-density inner membrane and hypo or iso-dense collections lateral to that indicates the risk of enlargement and recurrence as same as homogeneous type23,24).
3. Layered [separated or gradation]- 2 different density components were noted. A low-density component anteriorly and a high-density component posteriorly (Fig. 4) 23,24).
4. Trabecular [multilocular]- mixed density with high-density septations. Low risk of growth and recurrence (Fig. 5) 23,24).
Other less common forms of presentation of chronic subdural hematoma are bilateral ones, which generate a challenge to make the diagnosis by this means of imaging because although they can generate the effect of mass with a deviation of the midline when exerting opposing forces can neutralize, so this finding would not be as noticeable, a decrease in bilateral ventricular spaces can be found21). Likewise, the calcified or ossified subdural hematoma can be seen as an intracerebral subdural mass, composed of a hyperdense membrane that surrounds a hypodense centre in its internal and external parts25), also known as “armoured brain”, described as graded hematomas or bilateral hygromas26), hematomas in the posterior fossa are less frequent, however, they can be distinguished in images of the cervical spine20), Chronic subdural hematomas can present with associated infection, which can be seen in the tomographic image as regions of hyperintensity associated with the characteristic diagnostic isodensity27).
The use of nuclear magnetic resonance has increased over time due to its increased availability. Its use in this pathology is based on the study of possible differential pathologies. since for the diagnosis of chronic subdural hematoma the determination of age and diagnostic signs, it is more complex, and with less performance than tomography20), especially by the variability of the progression of oxidation of blood products. In this type of image, it is expected to find hyperintensity in T1 and hypointensity in T2, due to the concentration of proteins, an aspect that allows differentiation with cerebrospinal fluid, hyperintensity in fluid attenuated inversion recovery, diffusion weighted imaging restriction is absent in most hematomas, it can be found in infection or recent bleeding28). Likewise, in the presence of lesions of mixed densities, due to the risk of recurrence, the performance of NMR and the water diffusion variety has been evaluated, versus conventional tomography, regardless of availability, resonance has better performance than tomography for the detection of infarcts in recent stages, and prediction of treatment failure29). In the postoperative period, although the study of choice is still tomography, MRI is useful and should be considered initially in cases of suspected infarction or associated infection as possible complications depending on the clinical scenario20). As well, according to some studies, it is important to recall that the CT-Scan is a cost-effective method and preferred in daily clinical practice and can identify the size, thickness, midline shift, or even subdural clots, and the MRI can determine the internal anatomy and size of the CSDH7).
The patients undergoing surgery have been studied with tomography, and different studies propose postoperative volume as the greatest predictor of recurrence, beyond clinical or other imaging predictors. the greatest predictor supported by the literature is volume. it is proposed by 40/40 rule, which is when the volume is less than 40 ml or a volume less than 40% of the initial volume of cSDH, there is decreased risk of recurrence30), other studies propose a volume greater than 20 ml preoperatively as a risk factor for recurrence31), associated with or without midline deviation, but it is believed that the increase in size may be due to decreased intracranial pressure due to atrophy in elderly patients27). Other predictors studied are the characteristics of the hematoma, according to the Sakaguchi classification, a complex structure or membrane formation has been described as a possible predictor of recurrence27), hematoma density, and a direct relationship has been found between hyperintensity and mixed patterns with greater recurrence27).
Given the characteristics of this pathology, possible differentials are hygromas, defined as a collection of cerebrospinal fluid in the subdural space due to traumatic injury to the arachnoid or non-traumatic causes such as hypotension, dehydration, and atrophy, which cannot be easily distinguished from subdural hematomas. Since they are found as hypodense lesions in the subdural space, for which it is necessary to demonstrate the presence of blood products through images such as MRI20). Likewise, other differentials may be external hydrocephalus or the early postoperative period, lesions with similar characteristics but mostly isodense21), from subarachnoid hematomas, it differs from the involvement of the subarachnoid spaces in cisterns and grooves20). With meningiomas and metastasis of prostate cancer, their location in the subdural space and the characteristic hypodensity make it difficult to differentiate with chronic subdural hematoma, so in these cases, it is considered whether there is diagnostic suspicion to carry out a contrast study to make the differentiation32).
As suggested by Ragland and Lee2), the initial assessment must evaluate de ABC (airway, breathing, and circulation), the life-threatening hazards, the Glasgow Coma Scale (GCS) Score to identify the need for tracheal intubation, as well a clinical history of trauma, falls, antiplatelet or anticoagulant therapy usage should be clarified. If needed, laboratory studies should include the blood-cell count and coagulation test, electrolytes values, and liver function test.
In patients with minor symptoms (cSDH thickness <10mm with no or mild mass effect) conservative trial is considered. Asymptomatic small cSDH may undergo spontaneous resolution. Several pharmacological therapies33) have been tried as a part of the treatment regime as mentioned in Table 2. Symptomatic cSDH mostly land up in surgery, they very rarely undergo spontaneous resolution2). These patients need close clinical and radiological follow-up with prolonged discontinuation from anticoagulants and antiplatelets.
Surgical treatment is divided into preoperative, operative, and postoperative management.
Surgical treatment is recommended for symptomatic patients, even more, in those patients with neurological symptoms using burr-hole drainage (Table 3)5). It is usually recommended because, for some authors, as said by Vacca and Argento in their manuscript, its existence implies that the physiologic mechanisms are insufficient or unavailable to reabsorb the hematoma19). Even though, twist drill craniotomy and open craniotomy are also suggested for CSDH treatment19). Recent literature establishes that the usage of a drain after the CSDH drainage is associated with reduced recurrence and less mortality at a 6-month follow-up7). The CSDH is considered a reversible cause of dementia, drainage is related to independence of daily life activities and psychiatric function improvement, as well, early surgical interventions are most likely to be beneficial in the patient's prognosis7).
The membranes of the CSDH removal are still controversial due to the risk of damage to the underlying arachnoid surface and the capillaries, still, when these are calcified or thick, their removal might allow the re-expansion of the brain after the hematoma drainage7).
Three primary surgical techniques
1. Twist drill craniostomy
2. Burr hole craniostomy
3. Craniotomy
Popularized by Mark Walder in 1981. It is the most common technique performed so far. Two burr holes are placed one in the frontal and the other in the parietal region. The distance between the 2 boreholes should be at least 7 centimetres. One burr hole can be considered if the collection is more localized.
Used in hypodense collections with no membranes. Done under local anaesthesia at bedside or ICU. Yagnik et al. presented the results of a systematic review and meta-analysis of 16 articles comparing twist drill craniostomy and Burr hole drainage and found that complications, cure, recovery, and mortality rates were similar in the two groups34). Though there was an increased risk of recurrence of CSDH in twist drill craniostomy results with closed suction drainage in twist drill craniostomy were similar to Burr hole drainage (Fig. 6).
Subdural evacuating port system (SEPSTM) is a unique, patented technology that requires a relatively smaller size burr hole craniostomy (5 mm). SEPSTM is placed under local anaesthesia, providing a closed system in the extradural space without the need for irrigation or aspiration. It is suitable not only for the treatment of chronic and subacute subdural hematomas but also for subdural hygromas.
A craniotomy of the size of 6 centimetres or more. Reserved for patients with a significant acute component, multiple membranes, and recurrent chronic subdural haemorrhage. Here dura and outer membrane of the chronic subdural cavity are open and irrigated generously with saline. (mini craniotomy is less than 6cm) (Fig. 7) 35).
In general, performing craniotomy has less recurrence rate but high morbidity. Performing twist drill craniostomy has a high recurrence rate but less morbidity. Performing borehole craniotomy gives a balance between efficacy and risk. One surgical technique may not be appropriate for all CSDHs. The selection of an ideal treatment strategy for an individual patient should be targeted based on individual factors36).
Postoperative seizure prophylaxis is still discussed, however, it is sometimes recommended to use it for 7 days if the patient has an increased risk of seizures (alcohol consumption or traumatic brain injuries)2). According to some recent literature, from 11% to 19% of patients can present postoperative seizures, still, if the patient does not have a seizure history, it is not recommended the prophylaxis19).
Endovascular middle meningeal artery embolization with polyvinyl alcohol particles (PVA) is an emerging treatment for CSDH. It can be used for new or recurrent chronic SDH, or as prophylaxis to reduce the risk of recurrence after surgery. It is based on the principle that by blocking the blood supply to the membrane which has neovascularization the leakiness and fragility of these vessels are controlled which eventually leads to the cessation of the process of further CSDH formation37).
There is preliminary data to suggest that this minimally invasive therapy may be more efficacious and equally as safe compared to conventional, more invasive surgery37). In a case series by Link et al, 60 patients were treated with MMA embolization in which 41(91.1%) patients had stable or decrease in CSDH with avoidance of surgery and 4 (8.9%) patients had recurrence requiring surgical evacuation38).
In a study done by Ban et al where he compared 72 patients treated by MMA embolization Vs 469 patients treated by conventional means (surgically treated). It has been found that the treatment failure rate was 1.4% in the embolized group and 27.5% in surgically treated patients and surgical rescue was needed for 1.4% patients in embolized patients and 18.8% in the surgically treated group39).
In a meta-analysis conducted by Srivatsan et al among 9 studies published, it has been found that the recurrence rate of CSDH treated by embolization is 2.1%, but for surgical treatment, it was 27.7%40).
Catapano et al.41) did a retrospective propensity-adjusted comparison of MMAE Vs conventional treatment for 231 patients with CSDH. It has been found that MMAE is associated with good CSDH volume reduction and less treatment failure than conventional approaches.
Kan et al.42)did a multicenter prospective trial among 138 patients with CSDH in which 154 MMAE was done. 70.8% of patients had a greater than 50% reduction in hematoma volume while only nine patients (6.5%) required surgery.
In a recent systematic review and meta-analysis done by analysing database from 1987 to 2020 and by analyzing 20 studies by Ironside et al.43)it has been found that recurrence rate, surgical rescue and in-hospital complication was significantly low (718 MMAE Vs 698 conventionally treated patients).
This shows that MMA embolization is an emerging and promising minimally invasive treatment option for CSDH and may offer a safe and effective alternative to conventional surgery for specific patients, but it needs further randomized controlled trials for its definitive application.
According to some studies, even if it is scarce the literature reports, a calcified CSDH has been reported, its inside is from 0.3 to 2.7% according to Snopko et al.1). Calcified CSH is the blood collection localized under the outer shell of the brain 3 weeks after the injury1). Calcified CSDH is characterized by neurological symptoms with slow progression and brain atrophy found in neuroimages. It is important to consider that the differential diagnosis of this entity is subdural empyema, arachnoid cyst, epidural hematoma, or even a meningioma1). The calcified CSDH aetiology has been related to vascular factors, poor circulation, intravascular thrombosis, metabolic function, and metabolic events1). According to some case reports, conservative treatment is indicated in those elderly patients that do not present neurological symptoms (especially if there is a Calcified Chronic Subdural Hematoma), however, if there is a clinical deterioration is important to perform a complete resection of the calcified lesion1).
Another complication after the resection is the recurring haemorrhage of the subdural space, bleeding with brain compression, and adhered inner membrane dissection for the brain that will produce new neurological deficits. It is important to consider that patients could also present (in the 8% of cases according to the currently available literature, and as mentioned previously) acute-on-chronic SDH, this might be caused by head traumas and the clinical of the patient is characterized by the acute finding along with rapid neurologic deterioration. In the CT-Scan hyper and hypointensity are typical7). These patients have the worst outcome prognosis6).
Chronic subdural hematoma represents a unique challenge to the treating neurosurgical team. The evolution of diverse modalities of treatment has not brought a significant difference in the overall outcome of this indolent condition. Understanding the basic pathology and close monitoring of patients with chronic subdural hematoma points toward a better prognosis.
NOTES
Ethics statement
This study was a literature review of previously published studies and was therefore exempt from institutional review board approval.
Author contributions
Conceptualization: GAQO. Data curation: GAQO, VPM, DCSZ, RM. Formal Analysis: VPM, DCSZ. Investigation: DCSZ, EGB. Methodology: GAQO, DCSZ, LRMS, RM. Project administration: GAQO, BD, DCSZ, TJ, RM. Resources: GAQO, BD, DCSZ, EGB, RM. Software: DCSZ, TJ, RM. Supervision: VPM, LRMS, RM. Validation: VPM, DCSZ, LRMS, RM. Visualization: VPM, RM. Writing- original draft: GAQO, VPM, DCSZ, TJ. Writing, review & editing: GAQO, VPM, TJ, LRMS, RM.
Fig. 1.
Schematic diagram representing the events leading to the formation of Chronic subdural hematoma

Fig. 2.
Illustrative diagram showing the various layers of the skull and meningeal coverings of the brain.

Fig. 3.
Non-contrast pre and post-op CT scan head of a 55/male patient with homogenous, iso-dense chronic sural collection significant mass effect (black arrow). Note the reduction of chronic subdural hematoma collection in the post-op scan (yellow arrow). This patient underwent a single parietal burr hole with closed system drainage.
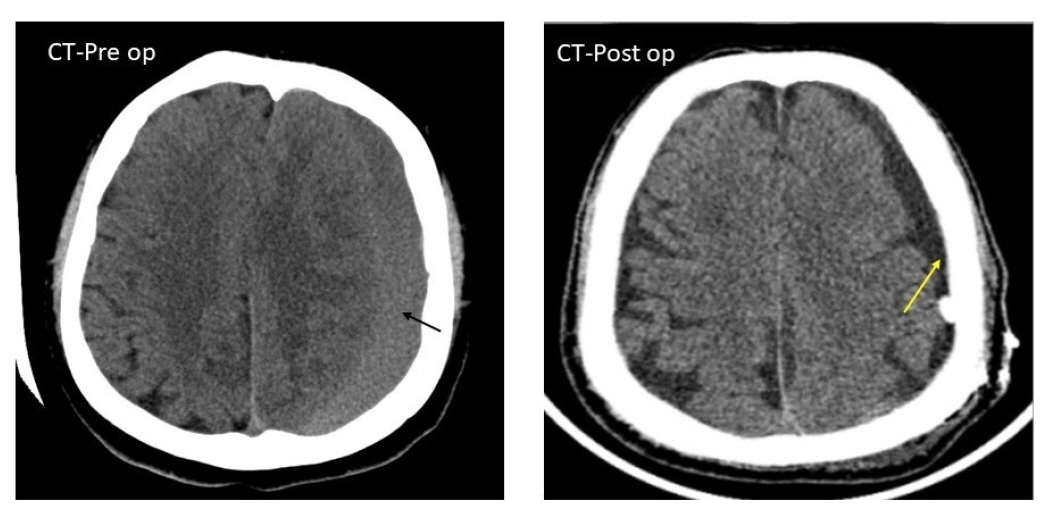
Fig. 4.
Non-contrast CT scan head showing a bilateral collection of the layered type of subdural hematoma. (A) The yellow stars suggest chronic subdural hematoma. (B) The red stars in the FLAIR sequence of the MRI brain suggest the subacute nature of subdural hematoma which indicates a layered type of chronic subdural hematoma.
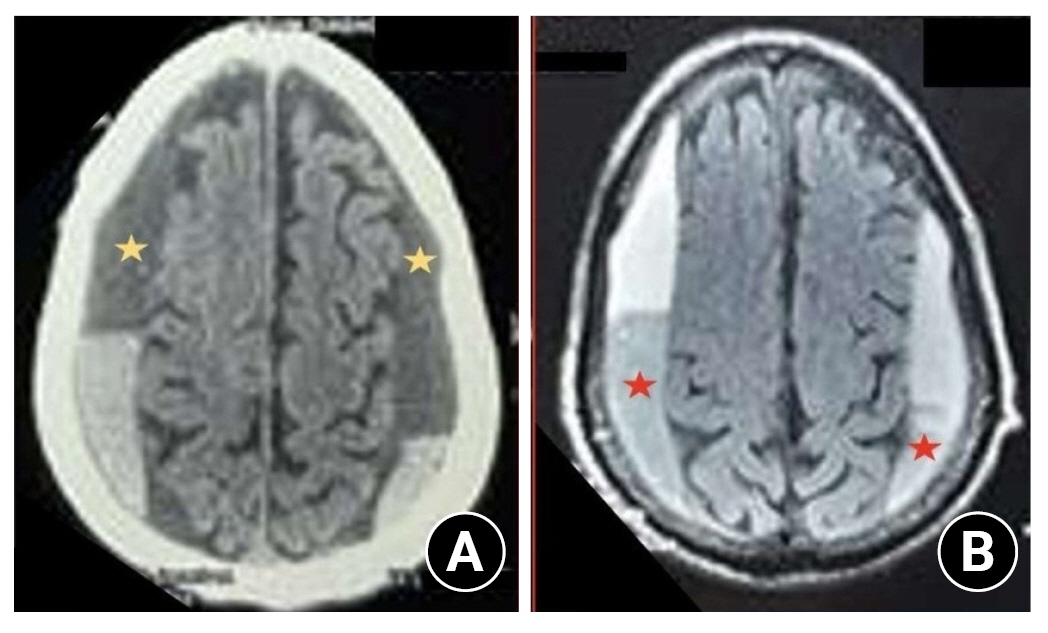
Fig. 5.
Shows the MR I(A) and CT scan (B) head of a 70/ male who underwent craniotomy for the trabecular (multiloculated) type of chronic subdural hematoma collection with significant mass effect.
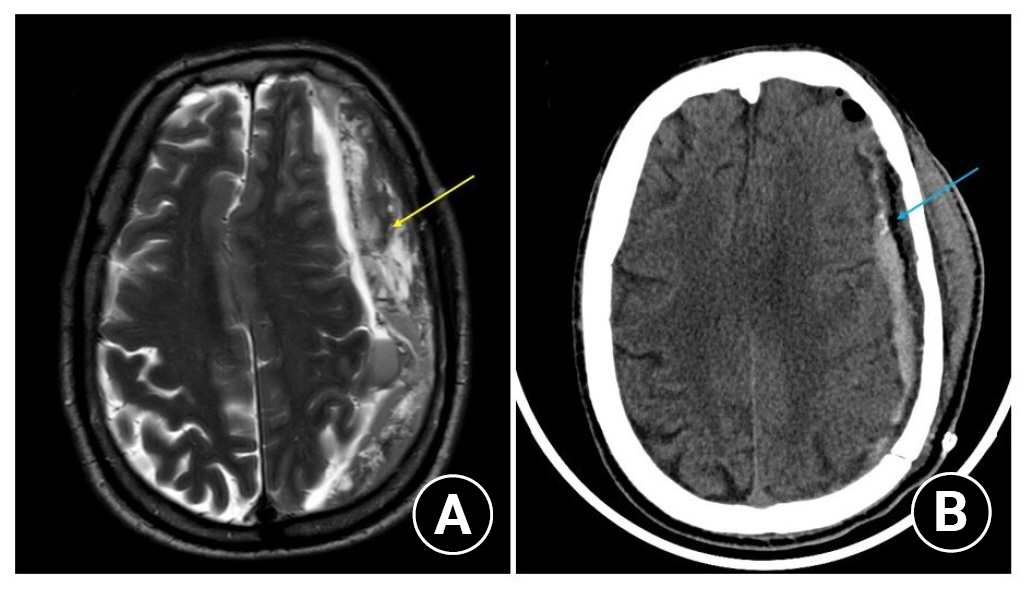
Fig. 6.
(A) shows twist drill craniostomy procedure. (B) shows efflux of chronic subdural collections.
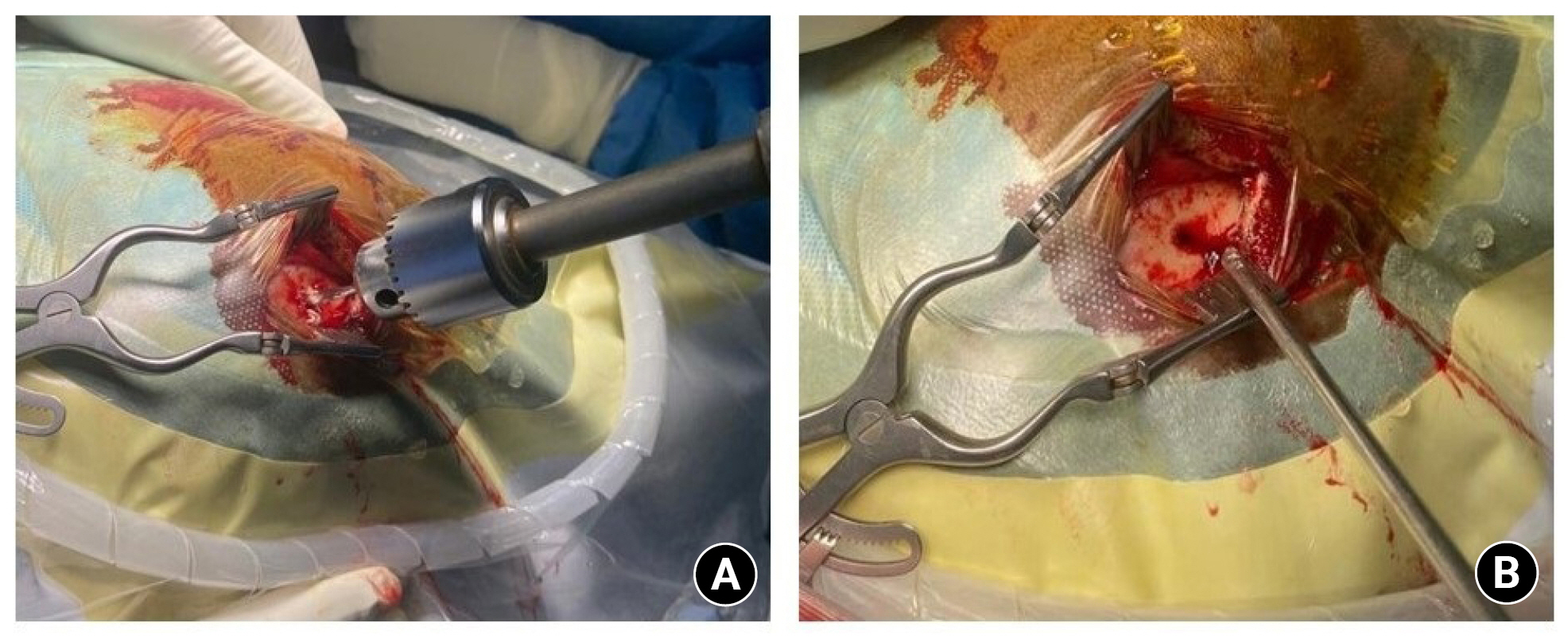
Fig. 7.
Shows the craniotomy procedure done for a chronic subdural hematoma. The thick outer membrane is demonstrated.
CSDH: chronic subdural hematoma.

Table 1.
Summarizes the advantages of two most commonly used pre op imaging
Table 2.
Pharmacological Treatment7
| Anticoagulant or Antiplatelet reversal therapy | Cessation of the therapeutic agents is the first step. |
| Prevent the hematoma expansion by giving reversal for anticoagulants thereby reduce operative risks when emergency neurosurgery is needed. | |
| Use of prothrombin complex concentrate, fresh frozen plasma, and Vitamin K is recommended for vitamin K antagonists. | |
| For newer oral anticoagulants [factor Xa inhibitors and direct thrombin inhibitors (Dabigatron)] there is no clear evidence for reversal of its effects. If there is no life-threatening condition or risk of hematoma expansion or need for urgent surgery then buying some time is the best form of reversal. | |
| For Dabigatran, FDA approved the usage of idarucizumab for its reversal. | |
| Antiplatelet effects last from 7 to 10 days so it is needed to wait for the replacement of the new and functional platelets but, when emergency surgery is needed- a platelet transfusion might be performed. | |
| Intravenous therapy | It is used to generate an osmotic gradient between the plasma and the brain by decreasing the water in the brain to decrease intracranial pressure. Hypertonic Saline Solution is recommended as it has an effect that helps in the modulation of the inflammatory response in the brain by reducing its swelling and edema thus avoiding states of intracranial hypertension and its related complications37). |
| Corticosteroids | Its usage has been related to a decreased rate of recurrence of the hematoma after surgical intervention. Generally not recommended due to lack of evidences, but there are ongoing trials Dex-CSDH, DECSA, and SUCRE33) can provide answers on its usage. |
| Anti-seizure therapy | recommended for high risk cases (eg; alcohol abuse). Its routine use in all cases is still under debate33) |
Table 3.
Surgical indications29)
Table 4.
Summary of surgical treatment
REFERENCES
1. Snopko P, Kolarovszki B, Opsenak R, Hanko M, Benco M. Chronic calcified subdural hematoma – case report of a rare diagnosis. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub 2020;164:209–212.


2. Ragland JT, Lee K. Chronic subdural hematoma ICU management. Neurosurg Clin N Am 2017;28:239–246.


3. Kolias AG, Chari A, Santarius T, Hutchinson PJ. Chronic subdural haematoma: modern management and emerging therapies. Nat Rev Neurol 2014;10:570–578.



4. Sabo RA, Hanigan WC, Aldag JC. Chronic subdural hematomas and seizures: the role of prophylactic anticonvulsive medication. Surg Neurol 1995;43:579–582.


5. Yang W, Huang J. Chronic subdural hematoma: epidemiology and natural history. Neurosurg Clin N Am 2017;28:205–210.

6. Uno M, Toi H, Hirai S. Chronic subdural hematoma in elderly patients: Is this disease benign? Neurol Med Chir (Tokyo) 2017;57:402–409.



7. Sahyouni R, Goshtasbi K, Mahmoodi A, Tran DK, Chen JW. Chronic subdural hematoma: a historical and clinical perspective. World Neurol 2017;108:948–953.

8. INGLIS K. Subdural haemorrhage, cysts and false membranes: illustrating the influence of intrinsic factors in disease when development of the body is normal. Brain 1946;69:157–194.


10. Gardner WJ. Traumatic subdural hematoma: with particular reference to the latent interval. Arch NeurPsych 1932;27:847–858.

12. Taguchi Y. [Prospects for conservative treatment of chronic subdural hematomas - investigation of the absorption process]. No To Shinkei 1982;34:999–1005.

13. Yamashima T, Friede RL. Why do bridging veins rupture into the virtual subdural space? J Neurol Neurosurg Psychiatry 1984;47:121–127.



14. Edlmann E, Giorgi-Coll S, Whitfield PC, Carpenter KLH, Hutchinson PJ. Pathophysiology of chronic subdural haematoma: inflammation, angiogenesis and implications for pharmacotherapy. J Neuroinflammation 2017;14:108.




15. Golden J, Frim DM, Chapman PH, Vonsattel JP. Marked tissue eosinophilia within organizing chronic subdural hematoma membranes. Clin Neuropathol 1994;13:12–16.

16. Killeffer JA, Killeffer FA, Schochet SS. The outer neomembrane of chronic subdural hematoma. Neurosurg Clin N Am 2000;11:407–412.


17. Yamashima T. The inner membrane of chronic subdural hematomas: pathology and pathophysiology. Neurosurg Clin N Am 2000;11:413–424.

18. Hong HJ, Kim YJ, Yi HJ, Ko Y, Oh SJ, Kim JM. Role of angiogenic growth factors and inflammatory cytokine on recurrence of chronic subdural hematoma. Surg Neurol 2009;71:161–165; discussion 165-166.


19. Vacca VM Jr, Argento I. Chronic subdural hematoma: a common complexity. Nursing 2018;48:24–31.
20. Carroll JJ, Lavine SD, Meyers PM. Imaging of subdural hematomas. Neurosurg Clin N Am 2017;28:179–203.


21. Mehta V, Harward SC, Sankey EW, Nayar G, Codd PJ. Evidence based diagnosis and management of chronic subdural hematoma: a review of the literature. J Clin Neurosci 2018;50:7–15.


22. Yadav YR, Parihar V, Namdev H, Bajaj J. Chronic subdural hematoma. Asian J Neurosurg 2016;11:330–342.



23. Nakaguchi H, Teraoka A, Suzuki Y, Adachi S. [Relationship between classification of CSDH according to the Internal architecture and hematoma contents]. No Shinkei geka. Neurological Surgery 2003;31:639–646.

24. Nakaguchi H, Tanishima T, Yoshimasu N. Factors in the natural history of chronic subdural hematomas that influence their postoperative recurrence. J Neurosurg 2001;95:256–262.


25. Turgut M, Akhaddar A, Turgut AT. Calcified or ossified chronic subdural hematoma: a systematic review of 114 cases reported during last century with a demonstrative case report. World Neurosurg 2020;134:240–263.


26. Viozzi I, van Baarsen K, Grotenhuis A. Armored brain in a young girl with a syndromal hydrocephalus. Acta Neurochir (Wien) 2017;159:81–83.



27. Miah IP, Tank Y, Rosendaal FR, Peul WC, Dammers R, Lingsma HF, et al, Dutch Chronic Subdural Hematoma Research Group. Radiological prognostic factors of chronic subdural hematoma recurrence: a systematic review and meta-analysis. Neuroradiology 2021;63:27–40.



28. Tamai S, Watanabe T, Ichinose T, Murakami KI, Ueno M, Munemoto S, et al. Morphological characteristics of infected subdural hematoma: comparison with images of chronic subdural hematoma. Clin Neurol Neurosurg 2020;194:105831.


29. Kochi R, Mino M, Sonobe S, Yoshida M, Tominaga T. Spontaneous development of encapsulated subdural hematoma in the posterior cranial fossa after cardiac surgery: a case report. NMC Case Rep J 2018;5:87–90.



30. Ridwan S, Bohrer AM, Grote A, Simon M. Surgical treatment of chronic subdural hematoma: predicting recurrence and cure. World Neurosurg 2019;128:e1010–e1023.


31. Altaf I, Shams S, Vohra AH. Radiolological predictors of recurrence of chronic subdural hematoma. Pak J Med Sci 2018;34:194–197.


32. Ganau M, Gallinaro P, Cebula H, Scibilia A, Todeschi J, Gubian A, et al. Intracranial metastases from prostate carcinoma: classification, management, and prognostication. World Neurosurg 2020;134:e559–e565.


33. Huang J, Gao C, Dong J, Zhang J, Jiang R. Drug treatment of chronic subdural hematoma. Expert Opin Pharmacother 2020;21:435–444.


34. Yagnik KJ, Goyal A, Van Gompel JJ. Twist drill craniostomy vs burr hole drainage of chronic subdural hematoma: a systematic review and meta-analysis. Acta Neurochir (Wien) 2021;163:3229–3241.



35. Krauss JK, Marshall LF, Weigel R. Medical and surgical management of chronic subdural hematomas. Youmans neurol surg 2011;6:535–543.

36. Santarius T, Kirkpatrick PJ, Kolias AG, Hutchinson PJ. Working toward rational and evidence-based treatment of chronic subdural hematoma. Clin Neurosurg 2010;57:112–122.

37. Link TW, Rapoport BI, Paine SM, Kamel H, Knopman J. Middle meningeal artery embolization for chronic subdural hematoma: endovascular technique and radiographic findings. Interv Neuroradiol 2018;24:455–462.




38. Link TW, Boddu S, Paine SM, Kamel H, Knopman J. Middle meningeal artery embolization for chronic subdural hematoma: a series of 60 cases. Neurosurgery 2019;85:801–807.



39. Ban SP, Hwang G, Byoun HS, Kim T, Lee SU, Bang JS, et al. Middle meningeal artery embolization for chronic subdural hematoma. Radiology 2018;286:992–999.


40. Srivatsan A, Mohanty A, Nascimento FA, Hafeez MU, Srinivasan VM, Thomas A, et al. Middle meningeal artery embolization for chronic subdural hematoma: meta-analysis and systematic review. World Neurosurg 2019;122:613–619.


41. Catapano JS, Ducruet AF, Nguyen CL, Cole TS, Baranoski JF, Majmundar N, et al. A propensity-adjusted comparison of middle meningeal artery embolization versus conventional therapy for chronic subdural hematomas. J Neurosurg 2021;135:1208–1213.









