 |
 |
- Search
| J Neurointensive Care > Volume 3(2); 2020 > Article |
|
Abstract
Objective
We investigated the features and possible predictors of deterioration in the acute or subacute phase of acute subdural hematoma (ASDH) patients aged > 65 years who initially received conservative treatment.
Methods
Between January 2016 and December 2019, 184 patients with ASDH were treated in a single institution. Eighty-two patients aged ≥ 65 years who had initially presented with preserved neurological status and conservatively treated were included in this study. We classified patients into deterioration and non-deterioration groups according to whether deterioration occurred in the acute and subacute phases. Medical records and computed tomography scans were reviewed, retrospectively.
Results
Twelve out of 82 patients (14.6%) exhibited deterioration in the acute or subacute phase; the remaining 70 patients had not deteriorated in the acute and subacute phases. Clinical outcome at discharge was significantly worse in the deterioration group (p<0.001) than in the non-deterioration group. The proportion of patients taking antithrombotics was significantly higher in the deteriorated patients (p=0.003). In the deterioration group, the midline shift was more severe (p<0.001), the hematoma was thicker (p=0.001), and the proportion of mixed-density hematomas was higher (p<0.001).
The conventional indication of surgery for acute subdural hematoma (ASDH) is widely accepted but, it does not specify the indication in elderly and the younger patients separately3). There have been many reports of unfavorable surgical outcomes in elderly ASDH patients even in those with an initially good neurological status2-4,17). To date, there is some uncertainty in making decisions for surgery in elderly ASDH patients18). Furthermore, in elderly patients, many comorbidities and expected complications of general anesthesia can impede the decision-making process. Before reconsidering whether the conventional indication of surgery is appropriate for elderly patients, the clinical course and outcome of conservative treatment in an elderly ASDH patient needs to be clarified. We investigated features and possible predictors of clinical deterioration in the acute or subacute stage in a specified patient population aged 65 years and over.
The Institutional Review Board approved this retrospective study and waived the requirement for informed consent. Between January 2016 and December 2019, 184 patients with ASDH were admitted to our institution. We made the decision of operation according to the conventional surgical indication. But, exceptionally, even with a maximal thickness of hematoma was ≥ 10mm and/or midline shift ≥ 5mm on the initial computed tomography (CT) scan, if the patients were on Glasgow Coma Scale (GCS) score ≥ 14 and did not show any neurological deficits except for mild to moderate degree headache, careful observation in the intensive care unit was planned. We excluded 32 patients who had undergone emergent surgery, 52 patients under 65 years of age, two patients who died immediately after visiting the emergency room (ER), two patients who could not undergo surgery because of unstable vital signs and seven patients who refused to surgery.Patients with deterioration caused by definite progression of associated trauma (n=4) and the main lesion on the initial computed tomography (CT) scan was associated injury (n=3) were also excluded. A total of 82 patients who initially exhibited good neurological status with not much ASDH and were thus determined to be managed conservatively were reviewed. We divided the phases based on the time from trauma onset, less than 4 days as the acute phase, 4 to 20 days as the subacute phase, and more than 21 days as the chronic phase, referring to a previous study9). Deterioration was defined as a decrease of ≥ 2 points on the Glasgow Coma Scale (GCS) scores, focal neurological deficits and medical treatment-resistant seizure. We classified patients into deterioration (DT) and non-deterioration (NDT) groups according to whether deterioration occurred ≤ 20 days from trauma onset. The DT group was classified into two subgroups, deterioration in the acute phase as an acute DT (ADT) subgroup and deterioration in subacute phase as subacute DT (SADT) subgroup. Age, sex, trauma mechanism, medical history, initial GCS scores in ER, platelet count; prothrombin time–international normalized ratio (PT-INR); activated partial thromboplastin time (aPTT); and modified Rankin Scale (mRS) scores at discharge were recorded. Unfavorable outcome at discharge was defined as mRS score of 4~6. Antithrombotic therapy were divided into six categories as single antiplatelet, dual antiplatelets, vitamin-K antagonist (warfarin), direct oral anticoagulants, other combinations of antiplatelet agents with anticoagulants, and no antithrombotics.
Maximal hematoma thickness, midline shift, density of hematoma, degree of brain atrophy, and combined lesions were assessed on initial and follow-up brain CT scans. Midline shift was measured as the maximal distance between the midline of the displaced neural structures and the center line of the skull. Compared with the brain gray matter, hematoma density was classified as homogenous if only high density of hematoma was distributed along the convexity, and mixed if high and low density were mixed in the hematoma, respectively. To evaluate brain atrophy, frontal horn index (FHI), cella media index (CMI), and sylvian fissure ratio (SFR) were recorded based on CT scans. FHI and CMI were calculated based on the method described by Meese14). We calculated FHI as the ratio of the greatest distance between the outer tables of the frontal bone to the maximal length between both external surfaces of lateral ventricle anterior horns5,14). CMI was recorded as the ratio of the greatest distance between both temporal bone outer tables at the cella-media level to the maximum length between lateral surfaces of both lateral ventricles on the same slice5,14). We modified the SFR measurement by referring to previous studies9,16). The ratio of the total maximal length of both Sylvian fissures to the maximal distance between the outer tables of temporal bones was recorded as SFR. If an intracranial lesion obliterated the cistern, the value of fissure on the opposite side was measured and multiplied by two16). In this study, the smaller the FHI value, the smaller the CMI value and the greater the SFR value, indicating the progression of brain atrophy.
Fisher’s exact test and chi-square test for categorical values, and a Mann-Whitney test for numerical values were performed to determine a value with a p<0.05 as statistical significance. The basic mean value and standard deviation (SD) values are also described. All analyses were performed with MedCalc (MedCalc Statistical Software version 19.1.7 (MedCalc Software Ltd, Ostend, Belgium; https://www.medcalc.org; 2020).
Twelve (14.6%) out of 82 patients deteriorated in the acute or subacute phase (DT group) and 70 (85.4%) patients (NDT group) did not undergo deterioration in the acute or subacute phase. Between the two groups, initial GCS scores (p= 0.20) were not significantly different. The mean (± SD) time from onset to deterioration was 5.0 ± 4.9 days. Two patients in the DT group (17%) could not undergo surgery due to unstable vital signs, while 10 (83%) underwent craniotomy or burr hole trephination. All patients in the DT group initially showed that CT findings were not consistent with definite indication of surgery and/or good GCS score of ≥ 14. Table 1 and Table 2 presents the baseline characteristics and clinical findings. In baseline characteristics, only premorbid antithrombotic medication (p=0.003) was significant for deterioration. Age (p=0.75), sex (p=0.36) and mechanism of trauma were not significantly different between the two groups. The medical history of diabetes mellitus (p=0.49), hypertension (p=1.00), coronary artery disease (p=1.00), atrial fibrillation (p=0.05), ischemic stroke (p=0.42), chronic kidney disease (p=0.10) and liver disease (p=1.00) did not show significant differences. There were no significant differences in the laboratory findings. However, mortality (p=0.002) and proportion of unfavorable outcomes at discharge (p<0.001) were higher in the DT group.
Table 3 describes the findings of the initial CT scan. In the DT group, the midline was more displaced (p<0.001) and the hematoma was thicker (p=0.001). Mixed density hematoma was more common in the DT group (p<0.001). Although the mean FHI and mean CMI were slightly higher and the mean SFR was slightly lower in the DT group, statistical differences were not identified in FHI (p=0.43), CMI (p=0.07), and SFR (p=0.84) values. There were no significant differences in the combined intracranial lesions.
Table 4 summarizes the characteristics of the 12 patients with deterioration. Six patients deteriorated in the acute phase and the other six patients deteriorated in the subacute phase. Time from onset to deterioration was 15.3 ± 14.7 (mean ± SD) hours in the acute phase and 223.2 ± 64.8 (mean ± SD) hours in the subacute phase, respectively. A decrease in GCS score ≥ 2 was observed in five patients of the ADT subgroup and in three patients in the SADT subgroup. One patient in the ADT subgroup showed intractable seizure. Focal neurological deficits without a decrease of GCS score ≥ 2 were observed only in the SADT subgroup (n=3). Evident rebleeding was identified all patients in the ADT subgroup. On the contrary, changes in hematoma density to the subacute stage were observed only in the SADT subgroup. The same number of patients in the two subgroups (n=4) showed unfavorable outcomes at discharge, and two patients in the ADT subgroup and one patient in the SADT subgroup expired due to deterioration.
According to a previous study, 6.5% to 23.2% of initially conservatively treated ASDH patients eventually experience worsening that required surgery in their clinical course1,16). A more recent study described that 9% of ASDH patients experienced worsening in the subacute phase9). Our study focused on elderly ASDH patients; 14.6% of initially non-surgically managed patients presented with deterioration on ≤ 20 days from trauma onset.
The patients in the DT group were more likely to have mixed density hematomas on the initial CT scan. Although the number of patients in the two subgroups was too small for statistical analysis, CT findings at the time of deterioration were different between the two subgroups. Even initial CT scans of two subgroups showed the same mixed density hematoma, it seems that the course of hematoma differs depending on the mechanism of development of mixed density. All cases in the SADT subgroup showed increased volume of hematoma with a change of density in a pattern similar to that presented in previous reports on subacute hematoma expansion (Fig. 1)9,16). An initially mixed density hematoma that is going to turn into a subacute expansion might be a mixture of cerebrospinal fluid with transudate, exudate from the dural membrane or concurrent ASDH combined with chronic hematoma11,16). However, in the ADT subgroup in our study, the increased hematoma volume on follow-up CT scan was not accompanied by a change of density, but rather resulted from evident rebleeding (Fig. 2). We interpreted that mixed density on the initial CT scan of the ADT subgroup might be an ominous signal of hyperacute hematoma expansion secondary to active bleeding caused by an injured intracranial vessel or antithrombotic-induced coagulopathy.
A recent meta-analysis reported that traumatic brain injury (TBI) patients aged ≥ 65 years who were taking antithrombotics had more chances of intracranial bleeding and poorer outcomes than those who were not on antithrombotics15). Therefore, prompt reversal of antithrombotic effects in patients prone to acute deterioration is considered safe. We routinely performed immediate cessation of antithrombotics; however this seems to be not enough during the acute phase. This is because it takes time to eliminate the antithrombotic effect completely. Furthermore, vulnerable patients such as the ADT group are exposed to the risk of antithrombotic-induced coagulopathy until the disappearance of its effect. Unfortunately, as of now, it seems unclear how to promptly eliminate the effect of antithrombotics6-8,10,12,15).
Tranexamic acid (TXA) is routinely administered for almost all patients with TBI in our institution. A recently published meta-analysis study reported that TXA administration tends to reduce mortality in TBI patients, and the occurrence of thromboembolic events was not significant19). At present, administration of TXA is may be an option for reducing risk of antithrombotic-induced bleeding tendency.
It has been expected that the atrophic brain is more prone to hematoma expansion9,16). A possible mechanism is that the wide subdural space itself results in an increased chances of vessel injury within its movement due to impacts13,16). In our study, although not statistically significant, it seems that brain atrophy tends to be more severe in the NDT group. These results are somewhat inconsistent with those previous studies but this is thought to reflect the compressed neural structures caused by hematoma or swelling of the acute phase. Because FHI and CMI use the values obtained from the lateral ventricles, if the ventricle is compressed due to hematoma, this will inevitably affect the results. And SFR can also be affected by combined lesion on the contralateral side or diffuse brain swelling.
This study has several limitations. Our study is based on a retrospective method with a relatively small number of cases in a single institution. Therefore, there may be an effect of patient selection bias. Even for the homogeneity of the study population and for focusing on more severe cases in acute settings, chronic SDH that may occur in the course of ASDH could not be evaluated. Moreover the antithrombotic type - related risk of deterioration could not be analyzed. Larger studies for investigating the clinical course of elderly ASDH patients and optimal management strategies are needed.
Acute or subacute deterioration occurred in 14.6% of non-surgically managed elderly ASDH patients. ASDH-related deaths and unfavorable outcomes were more common in the deterioration group. Close monitoring and extreme caution is necessary for non-surgically treated elderly ASDH patients, especially those taking antithrombotics, even if they presented with initially good neurological status.
NOTES
Fig. 1.
An 81-year-old male patient taking clopidogrel came to the emergency room (ER) 3 hours after tripping down the stairs. He was neurologically symptom free except for moderate degree headache. (A) A hematoma thinner than the skull is observed on brain CT taken 3 hours from trauma onset. The patient was admitted and the antiplatelet agent was stopped. There were no symptoms other than repeated headaches with improvement and aggravation. However, on the 10th day of the hospitalization, he complained of severe headache and drowsy mentality with a Glasgow Coma Scale (GCS) score 3/4/6. A follow-up CT scan showed hematoma enlargement along with a change in density and aggravated swelling with midline shifting. Emergent craniotomy was performed, and the hematoma was totally removed. The postoperative course was uneventful. The patient was discharged home a mRS 3 on the 15th day after surgery.

Fig. 2.
A 66-year-old female patient taking aspirin and clopidogrel was found lying drunk and was taken to the ER by the Emergency Medical Service. (A) Initial CT performed after 6 hours from the last normal time showed an 8mm-thick mixed density hematoma with minimal midline shifting. The patient showed no symptoms other than drowsy mentality of a GCS score 3/5/6 in the first assessment, so we decided to monitor the patient closely. However, 4 hours later, she deteriorated to a stuporous mentality of GCS score 1/1/5. (B) The follow-up CT showed the apparent enlargement of the hematoma and aggravated swelling. An urgent craniotomy and hematoma removal was performed. The postoperative course was uneventful and the patient was transferred to a rehabilitation hospital with a mRS 4 on the 26th day postoperatively.
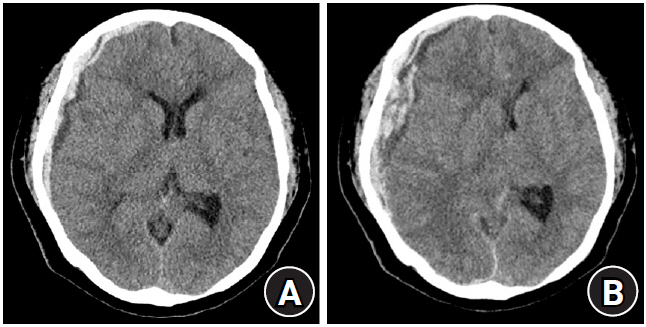
Table 1.
Baseline characteristics
| Characteristics | Non-deterioration (n=70) | Deterioration (n=12) | p value |
|---|---|---|---|
| Age | |||
| Mean (years) ± SD | 77 ± 7.3 | 77.3 ± 6.6 | 0.75 |
| Sex, n (%)* | 0.36 | ||
| Male | 35 (50) | 8 (67) | |
| Female | 35 (50) | 4 (33) | |
| Mechanism of trauma, n (%) | |||
| Slip | 36 (51) | 6 (50) | 0.93 |
| Fall | 11 (15.7) | 3 (25) | 0.42 |
| Traffic accident | 11 (15.7) | 0 (0) | 0.35 |
| Assault | 3 (4.3) | 0 (0) | 1.00 |
| Unknown | 10 (14.3) | 3 (25) | 0.39 |
| Other | 0 (0) | 0 (0) | N/A |
| Medical history, n (%) | |||
| Diabetes mellitus | 17 (24.3) | 4 (33.3) | 0.49 |
| Hypertension | 49 (70) | 9 (75) | 1.00 |
| Coronary artery disease | 5 (7.1) | 1 (8.3) | 1.00 |
| Atrial fibrillation | 7 (10) | 4 (33.3) | 0.05 |
| Ischemic Stroke | 11 (15.7) | 3 (25) | 0.42 |
| Chronic kidney disease | 2 (2.9) | 2 (16.7) | 0.10 |
| Liver disease | 3 (4.3) | 0 (0) | 1.00 |
| Antithrombotic therapy, n (%) | 0.003 | ||
| Single antiplatelet | 24 (34.3) | 7 (58.3) | |
| Dual antiplatelets | 3 (4.3) | 1 (8.3) | |
| Warfarin | 2 (2.9) | 0 (0) | |
| Direct oral anticoagulant | 3 (4.3) | 3 (25) | |
| Other combinations of antithrombotics | 0 (0) | 0 (0) | |
| No antithrombotics | 38 (54.3) | 1 (8.3) |
Table 2.
Neurological and laboratory findings
| Findings | Non-deterioration (n=70) | Deterioration (n=12) | p value |
|---|---|---|---|
| Initial GCS score | 13.91 ± 1.729 | 14.25 ± 1.71 | 0.20 |
| Duration from onset to deterioration | |||
| Mean (days) ± SD | N/A | 5.0 ± 4.9 | N/A |
| Laboratory findings | |||
| Blood platelet count (x 103/µl) | 203 ± 78.2 | 197 ± 59.7 | 0.95 |
| PT-INR | 1.12 ± 0.26 | 1.10 ± 0.295 | 0.68 |
| aPTT (sec ± SD) | 33.92 ± 4.58 | 35.12 ± 6.88 | 0.77 |
| Outcomes at discharge*, n (%) | |||
| Unfavorable outcome† | 10 (14.3) | 8 (66.7) | <0.001 |
| Cases of mortality | 0 (0) | 3 (25) | 0.002 |
Table 3.
Findings on initial computed tomography scan
| Findings | Non-deterioration (n=70) | Deterioration (n=12) | p value |
|---|---|---|---|
| Midline shift (mean ± SD) mm | 0.67 ± 1.46 | 4.10 ± 3.43 | < 0.001 |
| Hematoma thickness (mean ± SD) mm | 7.03 ± 4.31 | 10.94 ± 3.78 | 0.001 |
| Hematoma density, n (%) | < 0.001* | ||
| Homogenous | 51 (72.9) | 2 (16.7) | |
| Mixed | 19 (27.1) | 10 (83.3) | |
| Combined lesion, n (%) | |||
| Intraparenchymal hemorrhage | 21 (30) | 3 (25) | 1.00 |
| Subarachnoid hemorrhage | 32 (45.7) | 5 (41.7) | 1.00 |
| Skull fracture | 2 (2.9) | 2 (16.7) | 0.10 |
| Pneumocephalus | 0 (0) | 1 (8.3) | 0.14 |
| Intraventricular hemorrhage | 0 (0) | 1 (8.3) | 0.14 |
| Epidural hematoma | 12 (17.1) | 0 (0) | 1.00 |
| Brain atrophy | |||
| Frontal horn index | 3.57 ± 0.35 | 3.64 ± 0.40 | 0.43 |
| Cella-media index | 4.41 ± 1.12 | 4.81 ± 0.76 | 0.07 |
| Sylvian fissure ratio | 0.067 ± 0.03 | 0.063 ± 0.025 | 0.84 |
Table 4.
Characteristics of subgroups of deteriorated patients
| Characteristics | Deterioration on acute phase (n=6) | Deterioration on subacute phase (n=6) |
|---|---|---|
| Time from onset to deterioration | ||
| Hours (Mean ± SD) | 15.3 ± 14.7 | 223.2 ± 64.8 |
| Days (Mean ± SD) | 0.64 ± 0.61 | 9.3 ± 2.7 |
| Presentation of deterioration, n (%) | ||
| Decrease of GCS score ≥ 2 | 5 (83.3) | 3 (50) |
| Focal neurological deficits † | 0 (0) | 3 (50)* |
| Intractable seizure | 1 (16.7) | 0 (0) |
| Follow up CT scan findings, n (%) | ||
| Rebleeding | 6 (100) | 1 (16.7)‡ |
| Change of hematoma density | 0 (0) | 6 (100) |
| Outcomes, n (%) | ||
| mRS score 4~6 at discharge | 4 (66.7) | 4 (66.7) |
| Mortality case | 2 (33.3) | 1 (16.7) |
‡ A follow-up CT scan in the acute phase of this patient showed a slightly increased volume of hematoma compared to initial CT scan. But, this patient did not deteriorated in the acute phase. The CT scan in the subacute phase showed hematoma enlargement along with a change in density and aggravated swelling.
REFERENCES
1. Bajsarowicz P, Prakash I, Lamoureux J, Saluja RS, Feyz M, Maleki M, et al. Nonsurgical acute traumatic subdural hematoma: what is the risk? J Neurosurg 2015;123:1176–1183.


2. Benedetto N, Gambacciani C, Montemurro N, Morganti R, Perrini P. Surgical management of acute subdural haematomas in elderly: report of a single center experience. Br J Neurosurg 2017;31:244–248.


3. Bullock MR, Chesnut R, Ghajar J, Gordon D, Hartl R, Newell DW, et al. Surgical management of acute subdural hematomas. Neurosurgery 2006;58:S16–24; discussion Si-iv.



4. Bus S, Verbaan D, Kerklaan BJ, Sprengers MES, Vandertop WP, Stam J, et al. Do older patients with acute or subacute subdural hematoma benefit from surgery? Br J Neurosurg 2019;33:51–57.


5. Chrzan R, Glen A, Bryll A, Urbanik A. Computed tomography assessment of brain atrophy in centenarians. Int J Environ Res Public Health 2019;16:3659.



6. Fortuna GR, Mueller EW, James LE, Shutter LA, Butler KL. The impact of preinjury antiplatelet and anticoagulant pharmacotherapy on outcomes in elderly patients with hemorrhagic brain injury. Surgery 2008;144:598–603; discussion 603-595.


7. Ivascu FA, Howells GA, Junn FS, Bair HA, Bendick PJ, Janczyk RJ. Predictors of mortality in trauma patients with intracranial hemorrhage on preinjury aspirin or clopidogrel. J Trauma 2008;65:785–788.


8. Karni A, Holtzman R, Bass T, Zorman G, Carter L, Rodriguez L, et al. Traumatic head injury in the anticoagulated elderly patient: a lethal combination. Am Surg 2001;67:1098–1100.



9. Kayahara T, Kikkawa Y, Komine H, Kamide T, Suzuki K, Shibata A, et al. Predictors of subacute hematoma expansion requiring surgical evacuation after initial conservative treatment in patients with acute subdural hematoma. Acta Neurochir (Wien) 2020;162:357–363.



10. Kobayashi L, Barmparas G, Bosarge P, Brown CV, Bukur M, Carrick MM, et al. Novel oral anticoagulants and trauma: the results of a prospective American association for the surgery of trauma multi-institutional trial. J Trauma Acute Care Surg 2017;82:827–835.


11. Kuwahara S, Fukuoka M, Koan Y, Miyake H, Ono Y, Moriki A, et al. Diffusion-weighted imaging of traumatic subdural hematoma in the subacute stage. Neurol Med Chir (Tokyo) 2005;45:464–469.


12. Milan M, Schaefer A. Race against the clock: overcoming challenges in the management of anticoagulant-associated intracerebral hemorrhage. J Neurosurg 2014;121 Suppl:1–20.
13. Mathew P, Oluoch-Olunya DL, Condon BR, Bullock R. Acute subdural haematoma in the conscious patient: outcome with initial non-operative management. Acta Neurochir (Wien) 1993;121:100–108.



14. Meese W, Kluge W, Grumme T, Hopfenmüller W. CT evaluation of the CSF spaces of healthy persons. Neuroradiology 1980;19:131–136.



15. Scotti P, Seguin C, Lo BWY, de Guise E, Troquet JM, Marcoux J. Antithrombotic agents and traumatic brain injury in the elderly population: hemorrhage patterns and outcomes. J Neurosurg 2019;133:486–495.


16. Son S, Yoo CJ, Lee SG, Kim EY, Park CW, Kim WK. Natural course of initially non-operated cases of acute subdural hematoma : the risk factors of hematoma progression. J Korean Neurosurg Soc 2013;54:211–219.




17. Sufaro Y, Avraham E, Alguyn F, Azriel A, Melamed I. Unfavorable functional outcome is expected for elderly patients suffering from acute subdural hematoma even when presenting with preserved level of consciousness. J Clin Neurosci 2019;67:167–171.


- TOOLS






