 |
 |
- Search
| J Neurointensive Care > Volume 6(1); 2023 > Article |
|
Abstract
Background
Cerebral vasospasm is a prevalent complication in traumatic brain injury (TBI), particularly in cases of traumatic subarachnoid hemorrhage (T-SAH), for which monitoring and treatment policies remain undefined.
Methods
A study was conducted on 49 T-SAH patients with multiple traumas to investigate cerebral vasospasm following T-SAH. Participants underwent transcranial doppler (TCD) and brain CT angiography (CTA) upon hospitalization and subsequent follow-up within seven days. Vasospasm was diagnosed through a comprehensive evaluation of TCD, CTA, and symptoms, with risk factors analyzed accordingly. The initial clinical status was assessed using the Glasgow Coma Scale (GCS), modified Fisher scale (mFS), and Hunt-Hess grade (HHG), while examining various factors to identify underlying risks and evaluating overall body damage via the Injury Severity Score (ISS).
Results
Cerebral vasospasm was confirmed in 19 out of the total 49 patients, which is 38.8%. Furthermore, the characteristics that had a statistically significant correlation with the vasospasm group were low GCS (p=0.03, odd ratio=0.592) and high ISS (p=0.022, odd ratio=1.124). Moreover, patients with vasospasm exhibited worse prognoses based on the Glasgow Outcome Scale (GOS) and modified Rankin Scale (mRS).
Cerebral vasospasm as a complication of spontaneous subarachnoid hemorrhage has been studied for many years and the strategy of the treatment as well as the monitoring system were well established. Considerable numbers of article have reported that traumatic subarachnoid hemorrhage (T-SAH) was also associated with cerebral vasospasm which has high morbidity and mortality1-4). However, the optimal treatment and surveillance methods of cerebral vasospasm after T-SAH are yet established despite of its high incidence and morbidity1-5).
The purpose of this study was to investigate the incidence and risk factors associated with cerebral vasospasm after T-SAH. We utilized a combination of periodic follow-up with transcranial Doppler (TCD) and computed tomography angiography (CTA) in the neuro-intensive care unit. By tracking changes in blood flow velocities and vessel caliber over time, we aimed to identify potential risk factors of vasospasm, including patient demographics, initial severity of head injury, and radiographic findings. In addition, we investigated the extent of systemic trauma using the Injury Severity Score (ISS) to verify the correlation with the occurrence of cerebral vasospasm6). Since isolated head trauma is uncommon, we deemed that it would be helpful in predicting the occurrence of vasospasm comprehensively.
Existing studies report a wide range of incidences for vasospasm following T-SAH, ranging from 20% to 63%3,5,7). The aim of this study is to determine the extent of vasospasm incidence after T-SAH. Moreover, it hypothesizes that the incidence and severity of cerebral vasospasm increase as the degree of the initial injury becomes more severe, and the study aims to verify the validity of this assumption. As mentioned earlier, the severity of the initial head injury will be evaluated using Glasgow Coma Scale (GCS) and HHG, and the initial brain computed tomography (CT) findings will be analyzed to determine whether the presence of skull fracture, epidural hematoma (EDH), subdural hematoma (SDH), intracerebral hemorrhage (ICH), and intraventricular hemorrhage (IVH) is associated with vasospasm. Furthermore, this study aims to examine whether the patients' medical history, age, and mechanism of injury are related to cerebral vasospasm. The association between systemic damage, assessed by the ISS, and cerebral vasospasm will also be investigated. Through this research, we hope to provide evidence that can help guide the management of T-SAH patients in the intensive care unit by identifying precipitating factors for cerebral vasospasm in advance and focusing treatment on the prevention of cerebral ischemia.
In this retrospective study, the medical records of 49 patients diagnosed with T-SAH between July 31, 2019, and December 15, 2022, were reviewed. Non-contrast brain CT scans were performed upon hospitalization to identify any associated injuries, including skull fracture, EDH, SDH, ICH, and IVH. GCS, modified Fisher scale (mFS), Hunt-Hess grade (HHG), and ISS were recorded at the time of admission to evaluate the severity of clinical status and T-SAH. Each patient's past medical history, including hypertension (HTN), diabetes mellitus (DM), dyslipidemia, liver disease, heart disease, ischemic stroke history, and smoking history, were recorded for risk factor analysis. All patients received care in a dedicated trauma intensive care unit (ICU), and 12 patients underwent surgical procedures such as hematoma evacuation or decompressive surgery due to emergency indications. The patients who were not underwent TCD or CTA were excluded from the study. The reasons of absence of study were refusal of the patient, compromised kidney function, and life-threatening injuries that the risk of transfer patient to the CT room was too high (Fig. 1).
A baseline TCD exam was performed within two days of hospitalization, and CTA was conducted to confirm the baseline vasculature. Thereafter, vasospasm was evaluated by tracking TCD and CTA with an interval of at least three days, up to seven days. However, patients with difficulty in maintaining vital signs due to multiple organ damage or those who underwent frequent surgical procedures for concomitant injuries received supportive care focused on maintaining vital signs. TCD and CTA were subsequently performed as soon as possible when the patients became stable. Cerebral vasospasm was confirmed when the TCD mean value of the middle cerebral artery (MCA) exceeded 120 cm/sec and vasospasm was simultaneously identified through brain CTA8). A diagnosis of vasospasm on brain CTA was made only when a consensus was reached among one neuro-radiologist and two endovascular neurosurgeons, for a total of three specialists. TCD was used to measure both mean and maximum blood flow velocities (cm/sec) in the MCA. When vasospasm was diagnosed, appropriate treatment options were selected based on the patient's condition. In cases where traumatic cerebral hemorrhage appeared to be mostly under control, we started triple H therapy (Hypertension, Hypervolemia, Hemodilution). If the control of hemorrhage was uncertain, we chose volume expansion only, while allowing natural systolic blood pressure. In cases of severe vasospasm, intraarterial chemical angioplasty was performed. The treatment was individualized according to each patient’s clinical condition and response to therapy.
Patients with diagnosed vasospasm were compared to those without vasospasm by measuring the max and mean values of TCD at the time of diagnosis. The Glasgow Outcome Scale (GOS) and modified Rankin Scale (mRS) were used to assess neurological outcomes at the time of discharge.
Age, gender, damage mechanism, past medical history, initial GCS, mFS, HHG, ISS, head injury types (skull fracture, EDH, SDH, ICH, IVH) were compared to check the risk factors of the group diagnosed with vasospasm and those not. In addition, the average number of days and duration of diagnosis for the vasospasm diagnostic group were calculated. GOS and mRS were compared between vasospasm group and without vasospasm group. Comparison of numerical and categorical variables was performed, respectively, with the Student’s t-test and Chi-square test. Variables that demonstrated a statistically significant correlation with the variable of interest in the univariate analysis were incorporated into the multivariate model. For all calculations, the commercially available software IBM® SPSS Statistics 25 was used. The level of statistical significance was defined at p < 0.05.
Table 1 presents the patients’ demographics. Out of the total 49 patients, 19 belonged to the vasospasm group (38.8%), and 30 belonged to the group without vasospasm. The average age of each group was 53.21±20.20 years and 56.63±17.35 years, with male/female ratios of 15 (78.9%)/4 (21.1%) and 21 (66.7%)/9 (33.3%), respectively. Vasospasm was diagnosed in 38.8% of all patients with T-SAH. In terms of the mechanism of injury (fall down, slip down, and traffic accidents), traffic accidents were more frequent in the vasospasm group, with 6 (31.6%), 3 (15.8%), and 10 (52.6%) respectively, whereas occurred at the same frequency in the group without vasospasm.
There was no significant difference in past medical history between the vasospasm and without vasospasm groups. The P values for HTN, DM, dyslipidemia, liver disease, heart disease, ischemic stroke, and smoking were 0.36, 0.69, 0.19, 0.42, 0.55, 0.74, and 0.05, respectively, and all were confirmed to be p > 0.05, indicating no statistical significance.
There was a significant difference between the vasospasm group and without vasospasm group in terms of the initial GCS, mFS, and HHG, which reflect the degree of injury upon the patient's first admission. The values for GCS (8.32±3.73 vs. 13.43±1.57; p=0.001), mFS (3.11±0.875 vs. 2.40±1.10; p=0.023), and HHG (3.84±1.02 vs. 2.17±0.83; p=0.001) were significantly different between the two groups. These findings suggest that a lower GCS score, a higher mFS score, and a higher HHG are associated with a higher tendency for vasospasm. In addition, the ISS, which reflects the overall degree of initial injuries to the body, also showed a significant difference between the group with vasospasm and the group without vasospasm (3.84±1.02 vs. 2.17±0.83; p=0.001) (Fig. 2). With the results to the above, the more severe the initial injury, the higher the probability of developing vasospasm. Regarding the analysis of brain CT findings, skull fracture [14 (73.7%) vs. 7 (23.3%); p=0.001] was the only factor that could predict vasospasm. EDH [5 (36.3%) vs. 3 (10.0%); p=0.132], SDH [15 (78.9%) vs. 15 (50.0%); p=0.043], ICH [6 (31.6%) vs. 9 (30.0%); p=0.907], and IVH [5 (26.3%) vs. 6 (20.0%); p=0.606] were not selected as significant factors for vasospasm. The multivariate logistic regression model analyzed statistically significant factors that were determined through statistical analysis. As a result, it was found that initial GCS and ISS had a significant correlation with cerebral vasospasm, with a p value of 0.03 (odd ratio 0.592) and 0.022 (odd ratio 1.124), respectively.
The group with vasospasm demonstrated a statistically significant difference compared to the group without vasospasm in the areas of GOS (3.53±2.01 vs. 0.47±1.04; p=0.001) and mRS (3.11±1.52 vs. 4.80±0.61; p=0.001) at the time of discharge. These findings suggest that the clinical outcome at discharge is worse in the vasospasm group compared to the group without vasospasm. A description of the vasospasm group is presented in Table 2, which shows that 12 out of 19 patients (63.2%) in the vasospasm group received surgical treatment, whereas none in the group without vasospasm underwent surgery. Neurosurgical operations, such as decompressive craniectomy, hematoma removal, and Burr hole drainage, were included in the surgical options. Vasospasm was diagnosed on average at 4.68±4.14 days of hospitalization, and it took an average of 11.16±7.90 days to resolve, which is longer than in previously reported studies1,8). Prolonged vasospasm lasting more than 15 days was observed in five patients in the vasospasm group (17, 16, 33, 20, 26 days, respectively). Our hospital serves as the regional trauma center, and a large number of patients with multiple severe traumas are treated, which may explain the higher incidence of severe vasospasm in our study.
Table 3 summarizes the differences in transcranial Doppler (TCD) values between the group without vasospasm and vasospasm group. Both mean and maximum values were measured on the right and left sides of each patient, so the sample value was twice the number of patients. The initial maximum (118.26±38.53 vs. 86.73±21.08; p=0.001), initial mean (82.24±34.52 vs. 53.90±13.53; p=0.001), follow-up maximum (167.87±43.67 vs. 86.95±18.62; p=0.001), and follow-up mean (126.21±44.81 vs. 54.73±12.45; p=0.001) values showed significantly higher results in the vasospasm group based on statistical analysis (p=0.001) (Fig. 3).
Patients diagnosed with vasospasm were initially given oral nimodipine9). In cases where severe vasospasm was observed on CTA, intra-arterial chemical angioplasty was performed on five patients (Fig. 4). The main symptoms of vasospasm observed were headache (1 patient), pupil dilation (1 patient), mental changes (1 patient), seizures (5 patients), and motor weakness (11 patients).
The acute management of traumatic brain injury is usually focused on preventing hematoma expansion and reducing intracranial pressure. However, there have been many reports of delayed cerebral hypoperfusion leading to ischemic damage10-13). Previous studies have reported that cerebral vasospasm occurs frequently after T-SAH1-3,5,14). Nevertheless, unlike spontaneous SAH, the guideline of surveillance or treatment of cerebral vasospasm after T-SAH is not definitive due to the complexity of accompanying injuries in trauma. Particularly in patients with decreased consciousness, failure to properly detect cerebral vasospasm may result in irreversible brain ischemic damage and poor prognosis. Based on the significance of these results, the current study aimed to conduct an in-depth investigation of vasospasm in T-SAH patients.
In previous studies, efforts have been made to detect vasospasm early after T-SAH, including noninvasive TCD, perfusion brain CTA follow-up to confirm perfusion defects, and monitoring modalities such as intracranial pressure (ICP) and continuous electroencephalogram (EEG)3,8). Vasospasm after T-SAH typically occurs earlier than vasospasm after spontaneous SAH and tends to resolve earlier as well2). Kramer et al. reported that the vasospasm occurs around 3 days after traumatic SAH, with a duration of up to 3 days, whereas in spontaneous SAH, it occurs between 4-14 days after the hemorrhage, with a duration of approximately 7-21 days2). In our study, cerebral vasospasm was diagnosed at an average of 4.7 days after traumatic SAH, which differs slightly from the onset data reported by Kramer et al. Our study was conducted on patients with multi organ injuries accompanied with brain injury. In many cases, critical care was prioritized that CTA study was delayed.
In order to create a measures of detecting vasospasm after T-SAH feasible in any neurointensive care unit, we chose less invasive TCD and brain CTA. According to Georgios Tsivgoulis et al. and Andrew M. Demchuk et al., MCA TCD values are reported to be superior in terms of agreement, sensitivity, and specificity compared to TCD of other cerebral arteries (anterior cerebral artery, basilar artery, vertebral artery) when diagnosing cerebral vasospasm definitively through CTA or cerebral angiography15-17). Therefore, the tracking of the maximum and mean TCD values in MCA on both sides was chosen since the MCA has been reported to be useful in predicting cerebral vasospasm. Another study using TCD surveillance, the Lindegaard ratio was used to exclude hyperemia when the mean TCD value of the MCA was high4). On the other hand, Nestor R. Gonzalez et al. reported that the Lindegaard Ratio alone is insufficient in determine of vasospasm, thus, imaging modalities, such as brain CTA, should be considered in a comprehensive diagnosis of vasospasm18). We combined brain CTA, which enables direct diagnosis of the shape of cerebral artery. Therefore, omitting the Lindegaard ratio did not lower the diagnostic level, as we believe.
The aim of this study was to investigate the risk factor (the condition in which cerebral vasospasm may occur), incidence, timing, and clinical outcomes of vasospasm in patients with T-SAH. The findings showed that cerebral vasospasm is a common complication in TBI patients and can result in significant morbidity and mortality. Specifically, the study found that the incidence of vasospasm was 38.8% in the T-SAH cohort, which is consistent with the reported incidence in previous studies 2).
The results of our study indicate that the severity of traumatic brain injury (TBI) is a significant risk factor for the development of vasospasm, with a higher severity of GCS, mFS, and HHG at initial hospitalization demonstrating a statistically significant association with cerebral vasospasm. The mFS and HHG used in our study are commonly used indicators to predict the incidence of vasospasm and neurological outcomes in spontaneous SAH19). The mFS reflects the degree of injury based on brain CT, while the HHG reflects the neurological status based on patient symptoms. Therefore, we included these indicators in the data to reflect the initial severity of injury clinically as well as radiologically. Fawaz Al-Mufti et al. reported that low initial GCS score in TBI patients is a risk factor for vasospasm, and in patients with initial GCS scores less than 9, vasospasm is more likely to occur1). Additionally, Matthias Oertel et al. reported that the post-traumatic vasospasm group had significantly lower GCS scores, with an average of approximately 7 points4). The multivariate model analyzed statistically significant factors that were determined through statistical analysis. As a result, it was found that initial GCS and ISS had a significant correlation with cerebral vasospasm, with a p value of 0.03 (odd ratio 0.592) and 0.022 (odd ratio 1.124), respectively. These findings are consistent with previous research indicating that low GCS scores are associated with an increased incidence of cerebral vasospasm. Additionally, this study was able to provide new evidence for the correlation between cerebral vasospasm and a new indicator added in this study, the ISS, which represents multiple organ injury.
Our study did not find a statistically significant association between past medical history and vasospasm occurrence. These findings suggest that the initial degree of injury and neurological deficit have the most significant impact on the development of vasospasm, which is consistent with previous research1). Underlying factors such as HTN, young age, and tobacco use are known to increase the risk of vasospasm in patients with spontaneous SAH8). However, cerebral vasospasm that occurs after T-SAH generally has a faster onset and tends to resolve more quickly than that of aneurysmal SAH4,5,8). Therefore, it is believed that the initial injury severity has a greater impact on the development of cerebral vasospasm than the patient's preexisting medical risk factors.
It is important to note that our study has certain limitations, including its retrospective nature and reliance on medical records for data collection. Our study was also conducted at a single center, which could limit the generalizability of our results. Furthermore, the absence of a control group in our study may restrict our ability to make causal inferences about the association between T-SAH and vasospasm.
Multiple trauma patients can have various injuries including head, chest, abdomen, and extremities. However, previous studies on the occurrence of cerebral vasospasm after TBI or T-SAH have focused on the initial head injury and neurological deficits1-5,14). This study used the ISS as an indicator of the initial severity of systemic injuries and found a statistical correlation between ISS and the occurrence of cerebral vasospasm6). In cases of severe multiple trauma, excessive bleeding leading to coagulopathy and systemic inflammatory reactions may play a role of development of vasospasm. However, further research should be carried out to understand its true pathophysiology. The clinical significance of our study is noteworthy as it emphasizes the need for early detection and aggressive management of cerebral vasospasm in TBI patients, particularly those with severe TBI and systemic damage. In order to achieve the goal, clinicians could utilize TCD and imaging techniques such as CT angiography to detect early signs of vasospasm and initiate treatment on time. Moreover, our findings suggest that TBI patients with vasospasm may require more intensive monitoring and management to prevent delayed cerebral ischemia and improve functional outcomes. In summary, our study underscores the prognosis associated with cerebral vasospasm in T-SAH patients. Clinicians must be vigilant about early detection and management of vasospasm, particularly in TBI patients with severe TBI and severe systemic injury. Further research is warranted to explore the pathophysiology of cerebral vasospasm in TBI and to develop more efficacious management strategies for this condition.
In patients with traumatic SAH, 38.8% were found to have cerebral vasospasm. Moreover, through multivariate logistic regression analysis, it was confirmed that the risk factors for cerebral vasospasm were an initial low GCS and the presence of more severe accompanying injuries (as evaluated by the ISS). Cerebral vasospasm is a common complication that hinders recovery and worsens prognosis, requiring early appropriate surveillance and management. It is crucial to have an insight into the likelihood of cerebral vasospasm occurring in cases with more severe initial accompanying injuries and low GCS for effective early management. Additionally, this can be monitored with regular follow-up of TCD and brain CTA. It is necessary to adjust the interval of regular follow-up through further research in the future and identify appropriate treatment policies and pathophysiological causes.
Fig. 1.
The flow of patient selection and surveillance process.
SAH: Subarachnoid hemorrhage, TCD: Transcranial doppler (cm/sec), CTA: CT angiography.
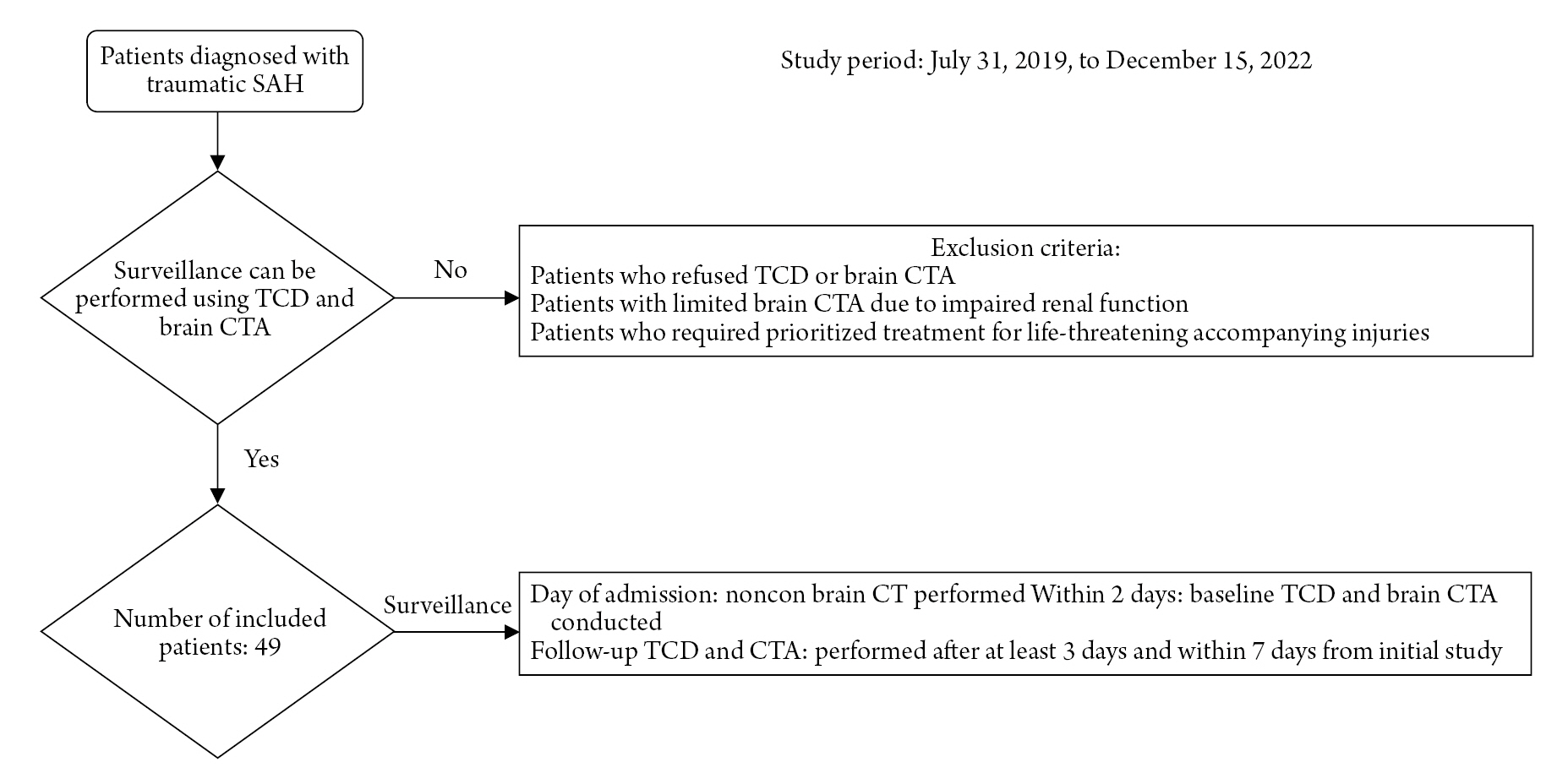
Fig. 2.
Difference in injury severity score (ISS) between the without vasospasm and vasospasm groups. *In the without vasospasm group, it represents a single patient who scored an ISS of 43. The vasospasm group showed a statistically significantly higher average ISS, but there was one patient in the without vasospasm group who had a notably high ISS yet did not experience vasospasm.

Fig. 3.
Difference in transcranial doppler value (cm/sec) between the without vasospasm and vasospasm groups.
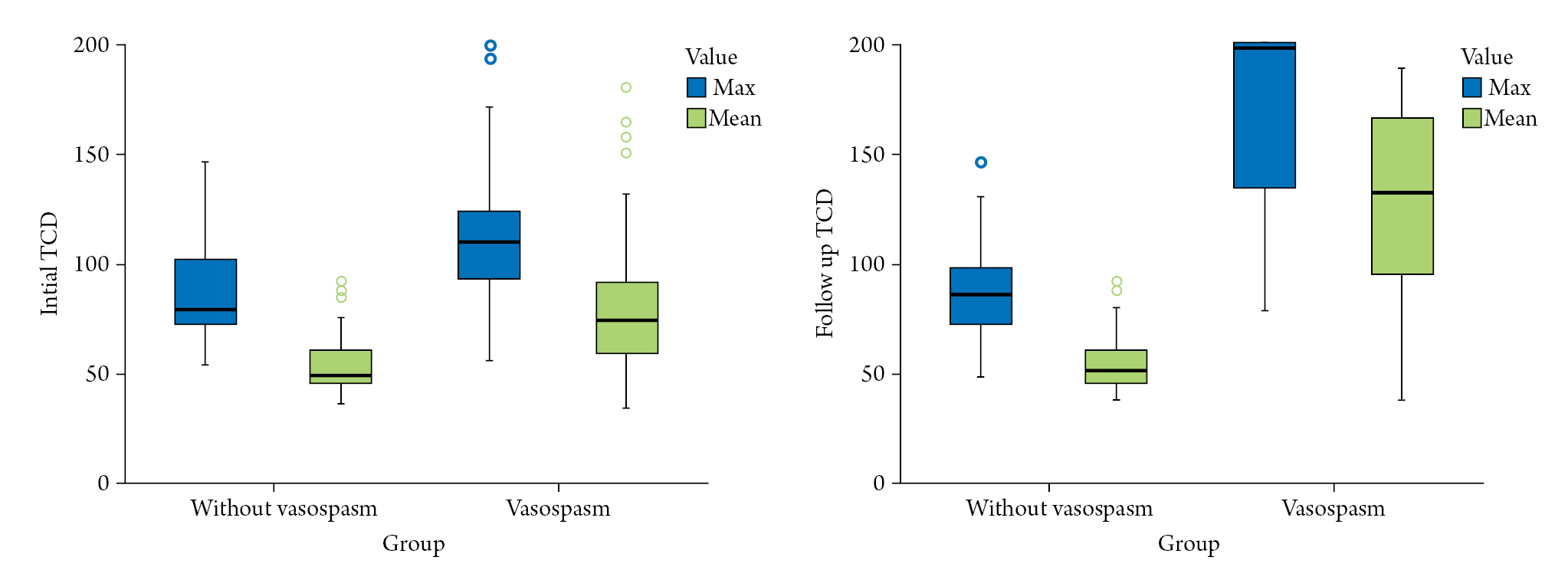
Fig. 4.
This patient underwent initial right internal carotid artery (ICA) embolization and sacrificed the right hemisphere due to Rt. ICA laceration. (A) Cerebral vasospasm causing low density in the left hemisphere was identified on brain CT (arrow). (B) Brain CT angiography revealed vasospasm of the left middle cerebral artery (MCA) (arrow). (C) Intra-arterial chemical angioplasty was performed five times, and the vasospasm improved (arrow). (D) Angiography showed vasospasm of the left MCA (arrow). (E) Angiography showed improvement of vasospasm on Lt. MCA (arrow).

Table 1.
Clinical characteristics of patients
Table 2.
Characteristics of the vasospasm group
Table 3.
Difference between the without vasospasm group and the vasospasm group
REFERENCES
1. Al-Mufti F, Amuluru K, Changa A, Lander M, Patel N, Wajswol E, et al. Traumatic brain injury and intracranial hemorrhage-induced cerebral vasospasm: a systematic review. Neurosurg Focus 2017;43:E14.

2. Kramer DR, Winer JL, Pease BA, Amar AP, Mack WJ. Cerebral vasospasm in traumatic brain injury. Neurol Res Int 2013;415813.


3. Maegawa T, Sasahara A, Ohbuchi H, Chernov M, Kasuya H. Cerebral vasospasm and hypoperfusion after traumatic brain injury: Combined ct angiography and ct perfusion imaging study. Surg Neurol Int 2021;12:361.



4. Oertel M, Boscardin WJ, Obrist WD, Glenn TC, McArthur DL, Gravori T, et al. Posttraumatic vasospasm: The epidemiology, severity, and time course of an underestimated phenomenon: A prospective study performed in 299 patients. J Neurosurg 2005;103:812–824.


5. Sander D, Klingelhofer J. Cerebral vasospasm following post-traumatic subarachnoid hemorrhage evaluated by transcranial doppler ultrasonography. J Neurol Sci 1993;119:1–7.


6. Pal J, Brown R, Fleiszer DJJoT, Surgery AC. The value of the glasgow coma scale and injury severity score: Predicting outcome in multiple trauma patients with head injury. J trauma 1989;29:746–748.

7. Armonda RA, Tigno TA, Hochheimer SM, Stephens FL, Bell RS, Vo AH, et al. Posttraumatic vasospasm and intracranial hypertension after wartime traumatic brain injury. Perspect Med 2012;1:261–264.

8. Izzy S, Muehlschlegel S. Cerebral vasospasm after aneurysmal subarachnoid hemorrhage and traumatic brain injury. Curr Treat Options Neurol 2014;16:278.



9. Chackupurakal R, Gebauer M, Dusse F, Hartmann A, Nakamura M, Wappler F, et al. An observational study on the effect of nimodipine on cerebral blood flow velocity and oxygenation in patients with subarachnoid haemorrhage. J Neurointensive Care 2021;4:1–5.


10. Borsotti F, Cossu G, Bartolini B, Daniel RT. Cerebral vasospasm following mild traumatic brain injury: A silent killer? Am J Med 2020;133:441–443.


11. Desai M, Morris NA. Prolonged post-traumatic vasospasm resulting in delayed cerebral ischemia after mild traumatic brain injury. Neurocrit Care 2018;29:512–518.



12. Fehnel CR, Wendell LC, Potter NS, Klinge P, Thompson BB. Severe cerebral vasospasm after traumatic brain injury. R I Med J 2014;97:45–46.
13. Ogami K, Dofredo M, Moheet AM, Lahiri S. Early and severe symptomatic cerebral vasospasm after mild traumatic brain injury. World Neurosurg 2017;101:813.e11–813.e14.


14. Zubkov AY, Lewis AI, Raila FA, Zhang J, Parent AD. Risk factors for the development of post-traumatic cerebral vasospasm. Surg Neurol 2000;53:126–130.


15. Demchuk AM, Christou I, Wein TH, Felberg RA, Malkoff M, Grotta JC, et al. Accuracy and criteria for localizing arterial occlusion with transcranial doppler. Am J Neuroradiol 2000;10:1–12.


16. Demchuk AM, Christou I, Wein TH, Felberg RA, Malkoff M, Grotta JC, et al. Specific transcranial doppler flow findings related to the presence and site of arterial occlusion. Stroke 2000;31:140–146.


17. Tsivgoulis G, Sharma VK, Lao AY, Malkoff MD, Alexandrov AVJS. Validation of transcranial doppler with computed tomography angiography in acute cerebral ischemia. Stroke 2007;38:1245–1249.


- TOOLS






