Large-Vessel Occlusion Stroke Associated with Covid-19: A Systematic Review and Meta-Analysis of Outcomes
Article information
Abstract
Background
SARS-CoV-2 induced respiratory illness is increasingly being recognized to be associated with neurological manifestations including an increase in the incidence of strokes, particularly those induced by large vessel occlusion (LVO). Given this, the aim of present study was to determine the influence of SARS-CoV-2 i.e. Coronavirus disease-19 (COVID-19) on mortality, neurological outcomes, and treatment response in patients with stroke due to large vessel occlusion induced by COVID-19.
Methods
A search of randomized controlled trials (RCTs), prospective and retrospective cohort studies was conducted through PUBMED, SCOPUS, MEDLINE, EMBASE, the Central Cochrane Registry of Controlled Trials, and CINAHL databases. The statistical analysis was performed using the relative risk with the Mantel-Haenszel methodology for dichotomous variables with a fixed-effects model. The Newcastle-Ottawa scale (NOS) was used to assess the quality of the publications and ROBINS-I tool was used to evaluate the risk of bias across the studies.
Results
Six retrospective observational cohort and case-control studies involving 1000 patients with LVO were included. The group of COVID 19 patients with LVO had a greater risk of mortality(OR= 7.09, [95% CI: 4.6-10.91], I2= 0%, p ≤0.00001), fewer rates of treatment success(OR 0.15 [95% CI 0.08-0.29], I2 = 49%, p ≤0.00001), and lower favorable outcomes (OR 0.39 [95% CI 0.16-0.96], I2 = 63%, p = 0.04) than COVID 19 negative patients with LVO.
Conclusion
The findings from present systematic review suggest that patients with COVID 19 and LVO stroke have higher mortality and poorer outcomes than COVID 19 negative patients with LVO stroke.
INTRODUCTION
Coronavirus disease-19 (COVID-19) had a global impact due to severe pulmonary involvement. Although infections and fatalities connected with this virus have reduced due to widespread vaccination, however there have been over 518 million confirmed cases and over 6 million deaths worldwide as of May 18, 20221). Even though COVID-19 has been mainly associated with respiratory sysmptoms, many neurological manifestations have been reported2,3); moreover, these manifestations have been linked to greater in-hospital mortality4). One of the main manifestations of this neurological presentation is acute stroke. The study by Misra et al. 5), which included 350 studies, providing data from 145,721 patients with COVID-19, found that one in 50 patients experienced a stroke. In particular, the increase in cases of large vessel occlusion (LVO) in patients with COVID-196) is noteworthy due to the increased risk of mortality and worst sixth-month good outcome (modified Rankin Scale score ≤2) that they present7).We conducted a systematic review and meta-analysis to assess the influence of COVID 19 on clinical outcomes and treatment success in patients with stroke due to occlusion of large vessels.
METHODS
Data source and study selection
A detailed search of randomized controlled trials (RCTs), prospective and retrospective cohort studies was conducted through PUBMED, SCOPUS, MEDLINE, EMBASE, the Central Cochrane Registry of Controlled Trials, and CINAHL until April 2022. The search strategy included subject headings (MeSH) and text words connected with Boolean terms, resulting in the following: (“Stroke” [Mesh term] OR “Ischemic stroke[Mesh term]) AND (“Large vessel occlusion” OR “large vessel stroke” OR “intracranial large vessel occlusion” [Mesh term]) AND (“COVID-19” OR “SARS CoV 2 Infection” OR “Coronavirus disease” [Mesh term]) AND (“RTC” OR “randomized clinical trial” OR “observational studies)
Inclusion criteria
The included studies were evaluated based on the following inclusion criteria: (1) RCTs, (2) quasi-RCTs (3) Prospective and retrospective observational studies comparing COVID 19 positive vs. COVID 19 negative patients (control group) with large vessel occlusion stroke.
Data extraction and quality assessment
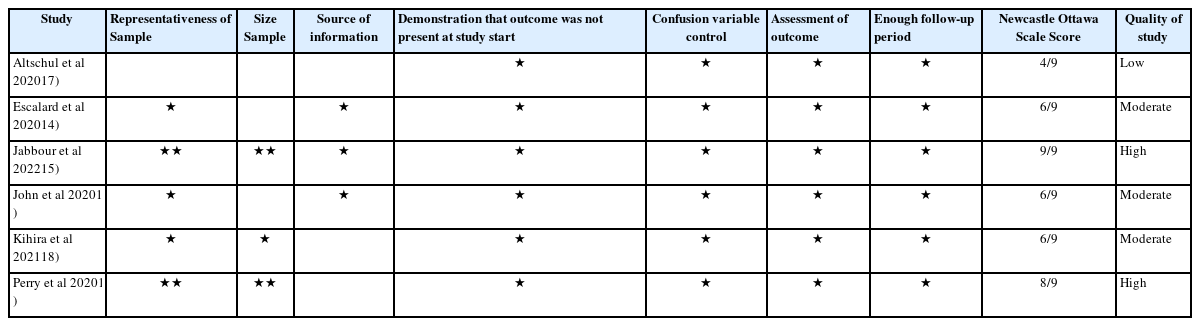
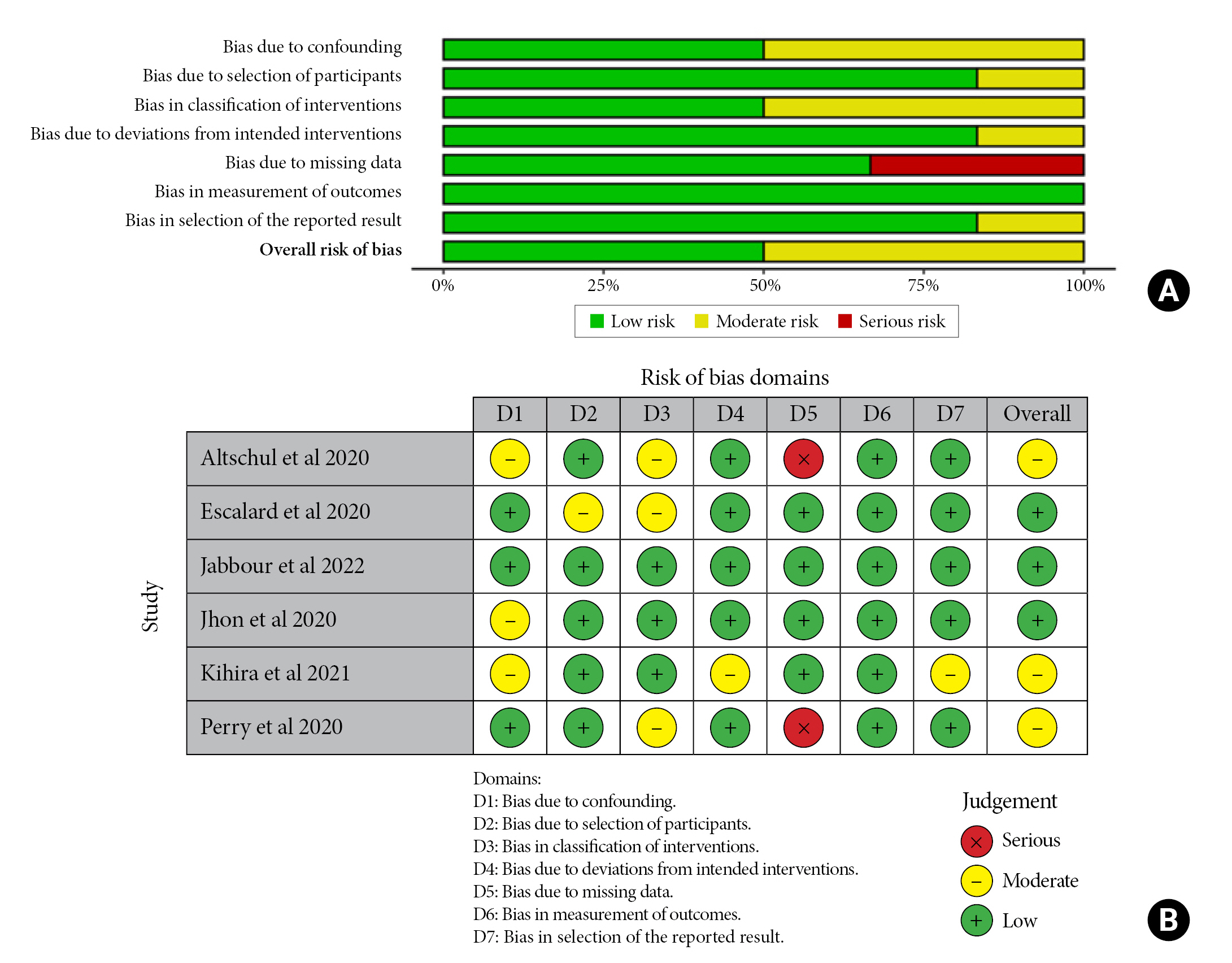
The quality of the included studies was evaluated using the Newcastle – Ottawa Quality Assessment Scale8). The criteria for determining high, moderate, and low methodological quality were as follows:eight or higher, six to seven, and five or less, respectively. To assess the risk of bias, ROBINS-I was used9).
Data analysis
Individually and separately, the following data were extracted: mortality, functional independence (modified Rankin scale 0 to 2, or Glasgow Prognostic Scale with a score of 4 or greater), treatment success (assessed by the rate of recanalization of occluded vessels), contact was made with the authors for missing data. The doubts were dispelled by consultation and consensus. The statistical analysis was performed using the relative risk with the Mantel-Haenszel methodology for dichotomous variables with a fixed-effects analysis model calculated using the Review Manager 5.3 software. Heterogeneity was determined by calculating Chi-square (I2), with a high level of heterogeneity among the included studies exceeding 65%.
RESULTS
Literature search results
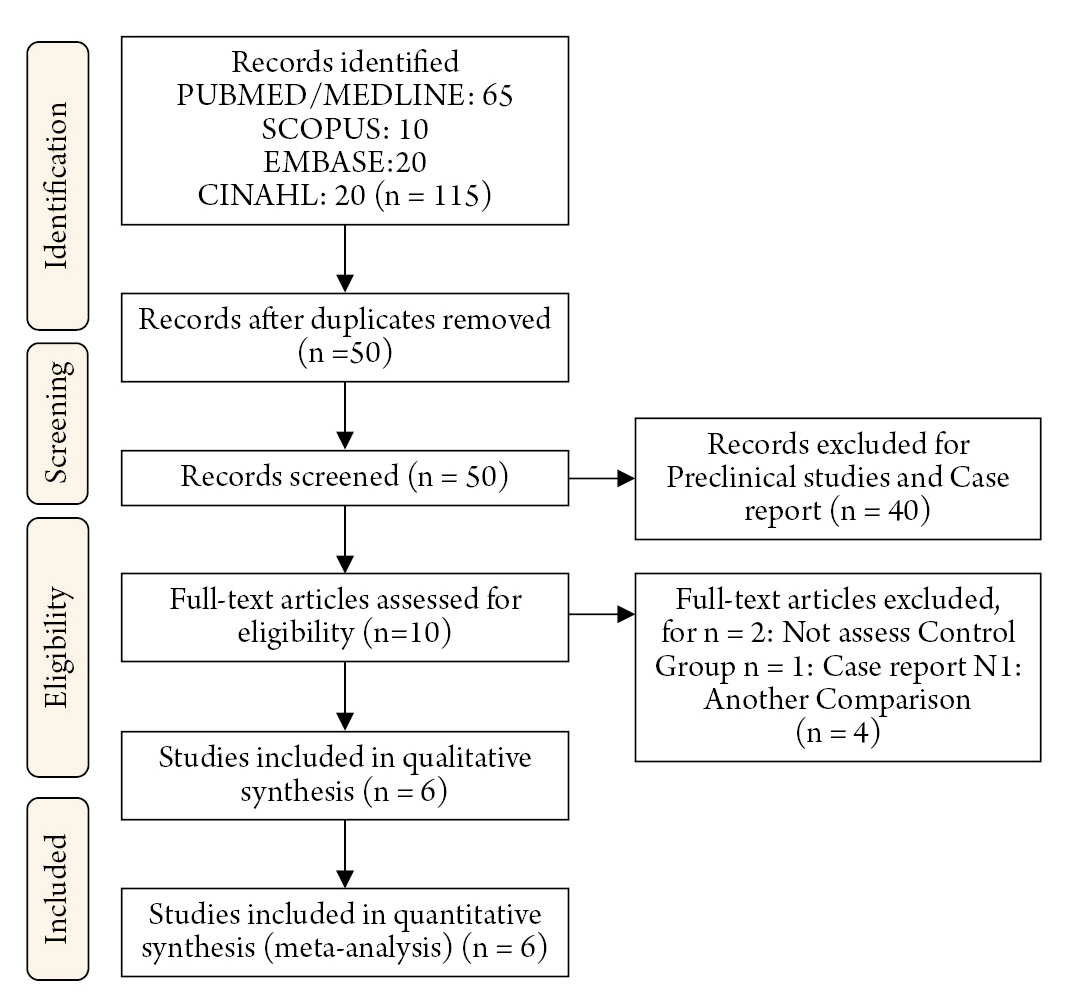
Following a comprehensive search for the information, 115 citations were found; after removing duplicates, screening titles, and abstracts, ten articles were chosen for full-text examination; finally, seven were retained. fourty studies were excluded during the screening phase as they were preclinical studies and case reports, and four were excluded during the full-text examination since two did not evaluate the control group10,11), one was a case report12), and one made another comparison13) (Tables 1 and 2; Fig. 1).
Risk of bias and quality assessment (Table 2)
All studies were categorized based on their risk of bias: three had a low risk14-16), and three had a moderate risk17-19) using the ROBINS-I tool. Two of the included studies17,19) exhibited a severe risk of missing data since they did not report the type of intervention for stroke revascularization. Simultaneously, three had a moderate risk in the cofounding domain16-18) and interventions classification14,17,19) (Fig. 2). Regarding the quality assessment, two studies were considered high quality15,19), three were of moderate quality14,16,18), and one was of low quality17). The latter was due to its non-representative sample size of 36 patients, 13 for the COVID group, and lack of a defined intervention protocol. In contrast, missing data could have influenced the outcomes of the studies conducted by Altschul et al., Kihira et al., and Perry et al., since the procedure of thrombolysis or endovascular thrombectomy might have altered the prognosis due to treatment-associated complications that were not recorded (Table 2).
Publication bias
Assessment of publication bias using the Funnel plot revealed asymmetry, consistent with publication bias; however, it was created using less than 10 studies, making the statistical analysis unreliable (Fig. 3).

Funnel plots. (A) Comparison: 1 Large Occlusion Vessel COVID 19 Positive Vs COVID 19 Negative, outcome: 1.1 Mortality. (B) Comparison: 1 Large Occlusion Vessel COVID 19 Positive Vs COVID 19 Negative, outcome: 1.2 Favorable Outcome. (C) Comparison: 1 Large Occlusion Vessel COVID 19 Positive Vs COVID 19 Negative, outcome: 1.3 Complete recanalization of occluded vessels.
Study characteristics
There was a total of six studies included (Table 3). One thousand participants with LVO stroke were eligible for the meta-analysis; of these, 292 had a positive PCR test for SARS-CoV-2, and 708 had a negative test. For each study, the total patient population, and its division into COVID-19 negative or COVID-19 positive patients with LVO were reported as the type of treatment received. Each study documented mortality and favorable outcomes, while the incidence of complete recanalization was reported in three of these studies.
Mortality
Mortality was reported in all included studies; 96 (32.87%) of 291 COVID-19 patientswith LVO died, compared to 40 (5.68%) of 706 patients in the COVID-19 negative LVO control group. The pooled estimate revealed a statistically significant difference (p ≤ 0.00001), demonstrating that the presence of LVO associated with COVID-19 increases 7.09 times the mortality compared to the COVID-19 negative LVO control group (OR= 7.09, [95% CI: 4.6-10.91], I2= 0%, Fig. 2).
Favorable neurological outcome
In the group of COVID-19 patients with LVO, 39 (13.35%) of 292 patients presented a favorable outcome, compared to 380 (53.82%) of 806 patients in the COVID-19 negative LVO control group; thisindicates that the favorable neurological outcome was lower in COVID-19 patients with LVO than the control group (OR 0.15 [95% CI 0.08-0.29] p ≤ 0.00001) with acceptable homogeneity (Chi2 = 9.84, I2 = 49%, Figs. 3-6).
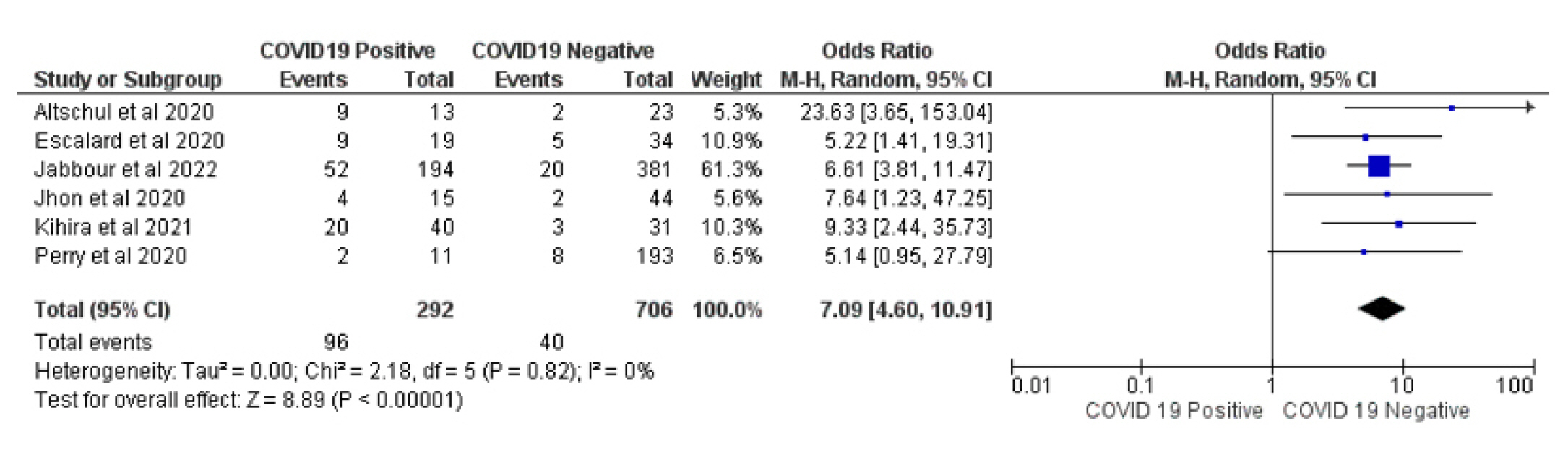
Forest plot of comparison: 1 Large Occlusion Vessel COVID 19 Positive Vs COVID 19 Negative, outcome: 1.1 Mortality.
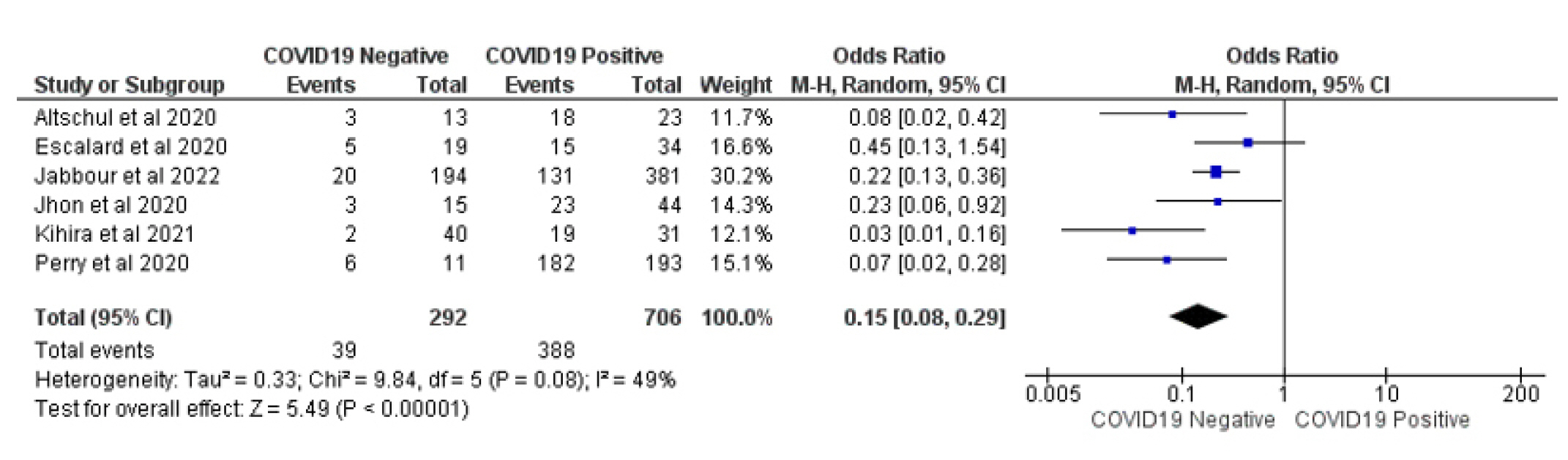
Forest plot of comparison: 1 Large Occlusion Vessel COVID 19 Positive Vs COVID 19 Negative, outcome: 1.2 Favorable Outcome.

Forest plot of comparison: 1 Large Occlusion Vessel COVID 19 Positive Vs COVID 19 Negative, outcome: 1.3 Complete recanalization of occluded vessels.
The success of stroke treatment was reported by 4 studies and was measured by the rate of full recanalization following revascularization therapy. 129 (47.75%) of 269 COVID-19 patients with LVO exhibited treatment success, compared to 269 (54.89%) of 490 patients in the COVID-19 negative LVO control group; this indicates that treatment was less effective in COVID-19 patients with LVO than in the control group (OR 0.39 [95% CI 0.16-0.96] p = 0.04) with high heterogeneity (Chi2 = 9.61, I2 = 63%, Fig. 4).
DISCCUSION
In this systematic review and meta-analysis, we aimed to assess the influence of COVID 19 on clinical outcomes and treatment success in patients with stroke due to occlusion of large vessels. The specific mechanism by which the virus causes strokes is not well known; however, it is thought that there are four processes responsible for its appearance; these include neuroinvasion, endotheliitis, ACE2 suppression, and hypercoagulable state. To begin, the SARS-CoV-2 has been identified in the brain tissue from autopsies of COVID 19 patients20); Song et al. 21). investigated the potential of SARS-CoV-2 to infect the brain using three different techniques; first, it was discovered that infected and adjacent neurons exhibited clear signs of infection and metabolic changes using brain organoids. Second, SARS-CoV-2 neuroinvasion was shown in vivo using mice overexpressing human Angiotensin-converting enzyme 2 (ACE2). Lastly, SARS-CoV-2 was discovered in cortical neurons during autopsies of COVID-19-related deaths. Although the exact mechanism by which neuroinvasion occurs is unclear, two mechanisms have been proposed. The first is through the neuronal pathways, where transneuronal transport of SARS-CoV-2 occurs via peripheral nerve endings22), particularly in the olfactory mucosa23,24). The second mechanism is the hematogenous route; as endothelial cells exhibit SARS-CoV-2 receptors, infection of mucosal linings may provide entry to the lymphatic system and circulation8,25); this route allows the virus to travel to several tissues, including the brain. Regarding endotheliitis, the virus has been found in brain endothelial capillaries of COVID 19 patients during the autopsy; furthermore, Stancu et al. 26). reported the case of an 81-year-old patient with endotheliopathy suggestive of endotheliitis with several strokes; all of this illustrates the virus's inflammatory effect on brain vessels, which can lead to endothelial dysfunction and strokes. This dysfunction is worsened by ACE2 deprivation caused by the virus, which, in conjunction with the hypercoagulable condition, contributes to the stroke's pathophysiology.
Through this study, it was demonstrated that COVID 19 patients with LVO had a greater mortality risk than the control group (p ≤ 0.00001), exhibiting statistical significance. Studies have shown that patients with COVID-19 and stroke had a higher frequency of large vessel occlusion and higher in-hospital mortality rate than stroke patients without COVID-1927).Subsequently, the results showed that favorable outcomes were lower in the COVID 19 patients with LVO than in the control group. This result is supported by Fabregas et al. study28 in which LVO and COVID-19 had a lower likelihood of achieving a favorable functional outcome than those who did not have COVID-19; however, this difference was not statistically significant (p = 0.079).
Regarding treatment success, it was shown that it was less effective in COVID-19 patients with LVO than in the control group 19(OR 0.39 [95% CI 0.16-0.96]; p = 0.04). However, even though sufficient recanalization indicates treatment success, this might result in poor results.In a study conducted by Escalard et al., ten COVID 19 patients with LVO were included, five of these patients got intravenous alteplase, and they all had thrombectomy; although nine of the ten patients had an effective recanalization, none exhibited early neurological improvement, four had early cerebral reocclusion, and six patients died in the hospital29).
Finally, concerning the limitations, we observed that there was a significant disparity in the number of patients in the control group and the COVID 19 patients with LVO, with the latter group having at least twice as many patients. In addition, as previously indicated, some studies did not report the treatment performed, which can cause alterations in the reported results.
CONCLUSION
In conclusion, this systematic review and meta-analysis demonstrated that patients with LVO had a greater mortality risk, fewer rates of treatment success, and lower favorable outcomes than patients with COVID 19 negative patients with LVO. The findings of this study will serve as a foundation for further research on the subject.
Notes
Conflict of interest
There is no conflict of interest to disclose.
Acknowledgements
We declare that we have no conflicts of interest, and we did not receive financial assistance.