Locked in Syndrome After Penetrating Traumatic Brain Injury
Article information
Abstract
We report a case of locked in syndrome after direct injury to the ventral pons following penetrating trauma. Locked in syndrome is a devastating and rare neurologic disorder that most commonly occurs after cerebrovascular accident involving the basilar artery. Traumatic etiology is rare but in previously reported cases has involved blunt vascular injury as the immediate cause. We present a case of a young male who suffered a penetrating wound to the pons that resulted in locked in syndrome. The diagnosis was confirmed by thorough physical examination, computed tomography and magnetic resonance imaging, and digital subtraction angiography. Locked in syndrome is an exceptionally rare entity in trauma. Prompt diagnosis requires careful physical examination and high clinical suspicion after appropriate mechanism of injury.
INTRODUCTION
Originally described in 1966, locked in syndrome is defined by quadriplegia, lower cranial nerve paralysis, and mutism with preservation of consciousness, vertical gaze, and upper eyelid movement. Incomplete variants of locked in syndrome have also been described which include additional voluntary movements. Cerebrovascular accident, whether infarction or hemorrhage, is the most common cause of locked in syndrome, making up at least 75% of all reported cases1). The exact incidence and prevalence of locked in syndrome has not been established due to its rarity, with most examples appearing as case reports or case series of fewer than 100 patients. Trauma appears as the causative etiology in 9-30% of locked in syndrome cases across several case series, making it the second most common etiology of this rare condition2) However, a review of the literature reveals that the majority of these traumatic cases of locked in syndrome are the result of blunt trauma leading to basilar or vertebral artery injury or brainstem hemorrhage3-5). One case of penetrating trauma from a high cervical gunshot wound (GSW) caused locked in syndrome after thrombosis of the basilar artery6) Herein, we present a case of traumatic locked in syndrome resulting from direct penetrating injury to the ventral pons.
CASE
A 30-year-old male presented to our urban level 1 trauma center after multiple stab wounds with a screwdriver including two to the right temporal region, one in the posterior neck, one in the back, and three in the left flank. In the field, the patient was hemodynamically stable, but was not moving any extremities. On arrival, the patient had a GCS of 11 (4E,1V, 6M), and although he was unable to move any extremity, his eyes were open, and he was able to blink and move his eyes when asked. He was intubated for airway protection.
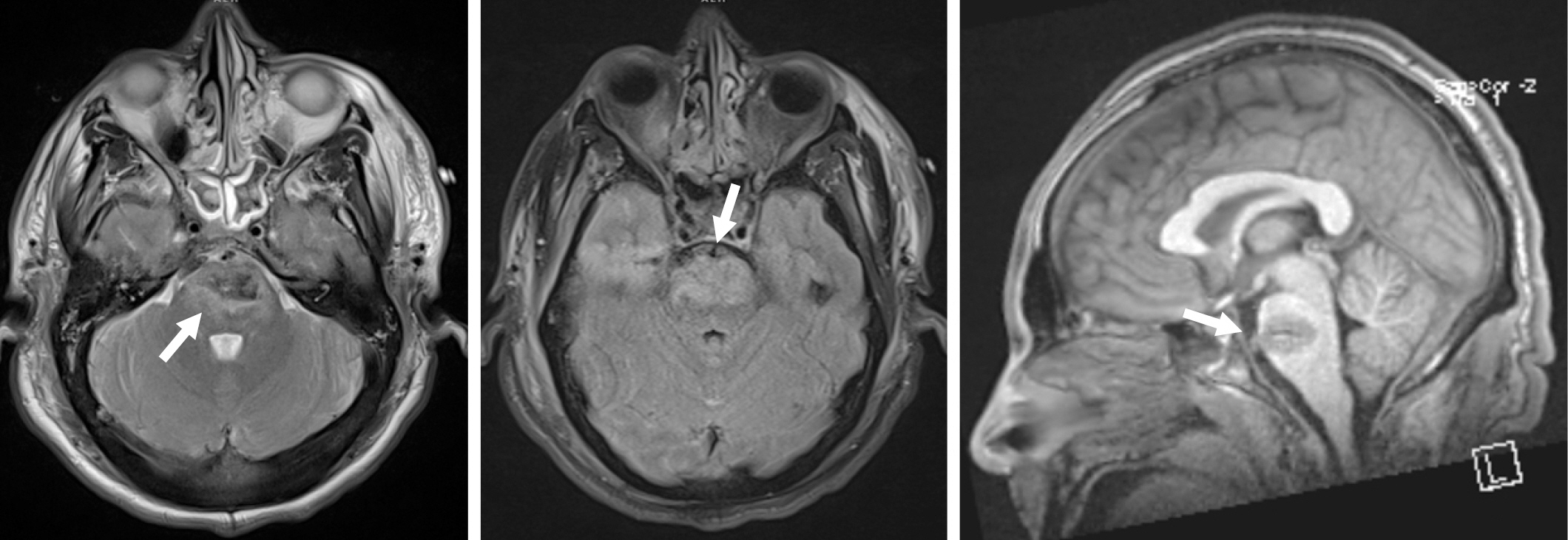
CT imaging revealed a penetrating injury with a displaced fracture through the squamosal portion of the right temporal bone. The injury tract extended through the right temporal lobe and into the pons, with small amounts of subarachnoid and parenchymal hemorrhage in the right temporal lobe and surrounding the pons and medulla. There was also parenchymal hemorrhage in the pons (Fig. 1). The cerebral vasculature was normal. MRI confirmed the CT findings with no evidence of superimposed territorial infarction (Fig. 2).
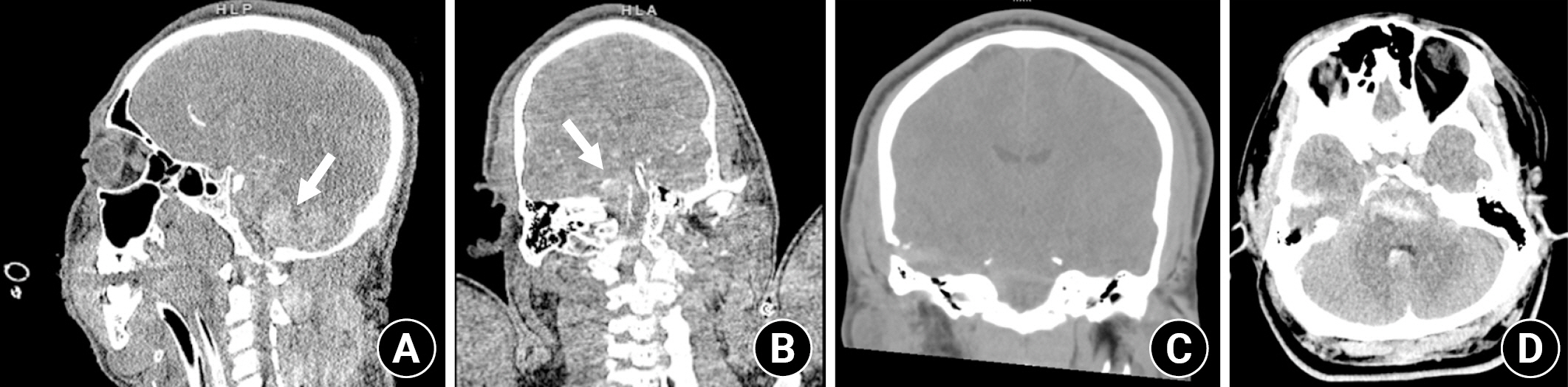
CT head obtained on presentation. (A, B) Arrows indicate subarachnoid and intraparenchymal hemorrhage in the pons and medulla (arrows). (C) Box shows wound tract through temporal lobe into the pons with adjacent hemorrhage. (D) Arrow indicates additional areas of hemorrhage around the brain stem.
The patient remained with a GCS of 11 following commands with blinking and eye movements and was diagnosed with locked in syndrome. He was treated with levetiracetam for seizure prophylaxis and broad-spectrum antibiotic prophylaxis. Digital subtraction angiography on hospital day two revealed a small focal irregularity in the mid basilar artery that was non-flow limiting (Fig. 3) which progressed to a moderate stenosis on repeat angiography four days later. This was treated with intra-arterial verapamil. (Fig. 4) His neurologic performance did not change, and he was deemed able to make decisions. On hospital day nine, he underwent percutaneous tracheostomy and percutaneous endoscopic gastrostomy tube placement under consent provided by the patient.

Digital subtraction angiography of left and right and basilar arteries obtained on hospital day 1 showing a left side dominance and a tiny, focal luminal irregularity of the mid basilar artery (P Com = posterior communicating artery).
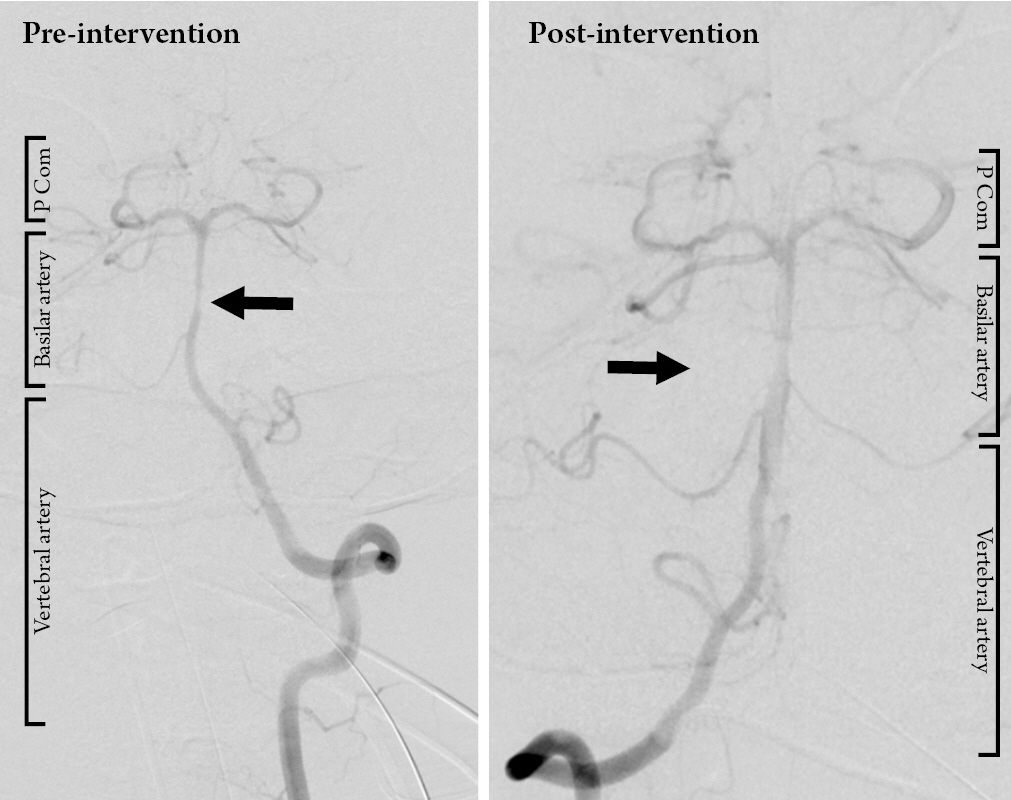
Digital subtraction angiography obtained on hospital day 5 showing vasospasm of the basilar artery (pre-intervention) and successful intra-arterial administration of verapamil via the left vertebral artery with improvement in the caliber of the basilar artery (post-intervention) (P Com = posterior communicating artery).
In neurorehabilitation, his function improved to a moderate to severe right hemiparesis where he was able to assist with transfers. He became able to manage his secretions and his tracheostomy was decannulated. His swallowing function improved, and he tolerated a mechanical soft diet with honey thickened liquids. Over the course of the next three months, he was able to eat a regular diet and he could dress and bathe himself. At the time of his discharge home, he was ambulatory with a cane for assistance. At about two years post-injury he continues to do well.
DISCUSSION
Locked in syndrome is a rare but usually devastating neurologic disorder that most commonly results from injury to the basilar artery from a primarily vascular pathology (ischemic or hemorrhagic stroke, thrombosis, or dissection). Although trauma is the second most common cause of locked in syndrome, we have been unable to find a previously reported case of locked in syndrome resulting from direct penetrating trauma to the brain stem. In the context of patient care within a trauma system, the diagnostic challenge presented by this condition is considerable due to its rarity, associated traumatic brain injury, and the subtlety of the physical examination findings required for diagnosis. In patients with locked in syndrome, the motor component of the GCS can only be calculated by using the patient’s eye movements, as no extremity movement is possible. The diagnosis of locked in syndrome is therefore often delayed. A high degree of suspicion and appropriate consultation with specialists will prevent delays in diagnosis and improve patient care.
Management of patients with locked in syndrome requires an extensive multidisciplinary approach including neurorehabilitation and specialized equipment and resources. Long term follow-up data from several studies confirms that varying degrees of neurologic recovery occurs, but most commonly, patients become dependent on their caregivers for most activities of daily living7-9). The ten-year survival rate has been reported as high as 80%.9 The majority of patients were able to return to living at home and had some degree of motor recovery which facilitated increased independence and improvements in quality of life. Over an 11-year period, just under 50% of patients considered euthanasia at some point. However, at the end of the follow up period, only one patient was still considering it8).
A significant body of work exists evaluating quality of life and functional recovery and the majority of interventions for these patients are focused on assistive devices, rehabilitation, and quality of life. Long term follow up data supports the idea that even with some degree of recovery, a significant number of patients with locked in syndrome are satisfied with their quality of life. Improvements in communication, often with the help of assistive technology, seem to be a primary driver of these quality-of-life improvements. The prognosis for major motor recovery has previously been reported to be minimal, however, Casanova et al found a significant motor recovery rate in 21% of patients within 3 to 6 months7,9). A near complete neurologic recovery, as experienced by our patient, is uncommon but has been previously reported10). Unfortunately, due to limited case numbers, there has been no consensus about the predictive power of imaging studies. However, in a review of the literature, non-vascular etiologies and incomplete locked in syndrome, as defined by any additional motor function preservation have been associated with more favorable neurologic recovery. This is likely due to a more limited pontine lesion. Our patient did have multiple digital subtraction angiography images confirming patent basilar arterial flow which supports the prior literature regarding prognosis for recovery.
The chronic nature of this condition, and the limited understanding of prognostication and recovery places a significant burden on the caregivers and support system of these patients, which can often lead to implicit bias from the health care team about what quality of life means for patients who are locked in and influence conversations with the patient and their family regarding medical decision making early in the acute care of this condition when the ability to recover and one’s future quality of life is unpredictable. It is therefore our hope that this report will improve timely recognition of this rare entity in trauma and illustrate the possibility for significant recovery to further improve understanding of this condition and thereby improve patient care.
Notes
Conflict of interest
There is no conflict of interest to disclose.