Neurosurgical Complications in Patients with Nasopharyngeal Cancer Accompanied by Skull Base Erosion: A case report
Article information
Abstract
A 66-year-old patient with nasopharyngeal cancer who had received chemotherapy and radiation therapy visited our emergency center with severe epistaxis. The patient was followed up with internal carotid artery trapping after a pseudoaneurysm was revealed in computed tomography angiography. Cerebrospinal fluid leakage was noted during follow-up observation after the intervention. This case presents various neurosurgical complications that can occur in patients with nasopharyngeal cancer.
INTRODUCTION
Radiation therapy is effective for the treatment of nasopharyngeal cancer due to the high radiation sensitivity of tumors1-3). Common complications that occur after radiation therapy include xerostomia and swallowing impairment; however, neurosurgical complications such as brain abscess and carotid artery stenosis have also been reported2). Furthermore, radiation therapy can cause vasculopathies such as moyamoya phenomenon and aneurysm formation4). Cerebrospinal fluid (CSF) leakage due to radio-induced osteonecrosis is also noted1). However, the role of a neurosurgeon in the treatment of complications is limited compared to that of an otolaryngologist or an oncologist. We report a case of a patient with nasopharyngeal cancer with complications requiring neurosurgical intervention.
CASE REPORT
A 66-year-old female patient with nasopharyngeal cancer visited the hospital with severe epistaxis(12/01/1). The patient had received radiotherapy in 2000 and total maxillectomy in 2013. A recurrence was found in magnetic resonance imaging (MRI) conducted due to the presence of a symptom of sixth cranial nerve palsy during follow-up observation, which was further confirmed by biopsy. Concurrent chemoradiotherapy (07/26/21-9/1/21) was performed with no therapeutic effect; consequently, palliative chemotherapy (10/24/21~11/24/21) was executed due to recurrent nasopharyngeal cancer with skull base involvement including bilateral carotid canals (Fig. 1). During 1L2C palliative chemotherapy, the patient presented symptoms of massive epistaxis accompanied by hypovolemic shock. An early aneurysm was suspected at the left cavernous internal carotid artery (ICA) on MRI (Fig. 2A), but the patient was discharged from the hospital because otolaryngology examination showed absence of bleeding. However, severe epistaxis was reconfirmed immediately after discharge. Computed tomography (CT) angiography showed increased size of the aneurysm despite the short interval (Fig. 2B). Digital subtraction angiography showed an inferior projecting aneurysm with lobulated configuration arising from the left cavernous ICA (Fig. 3).
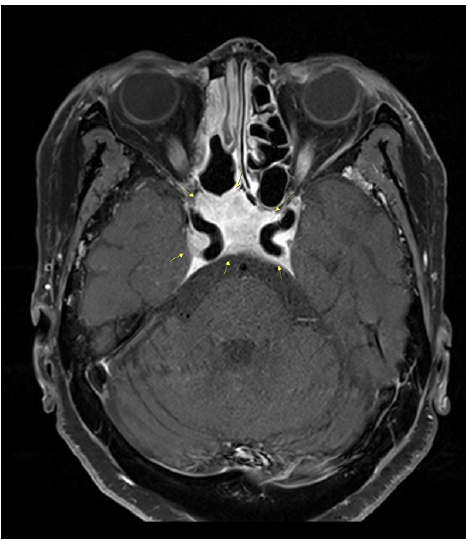
T1-weighted contrast enhanced magnetic resonance imaging. Contrast enhancement is observed in the skull base, including in both carotid canals.
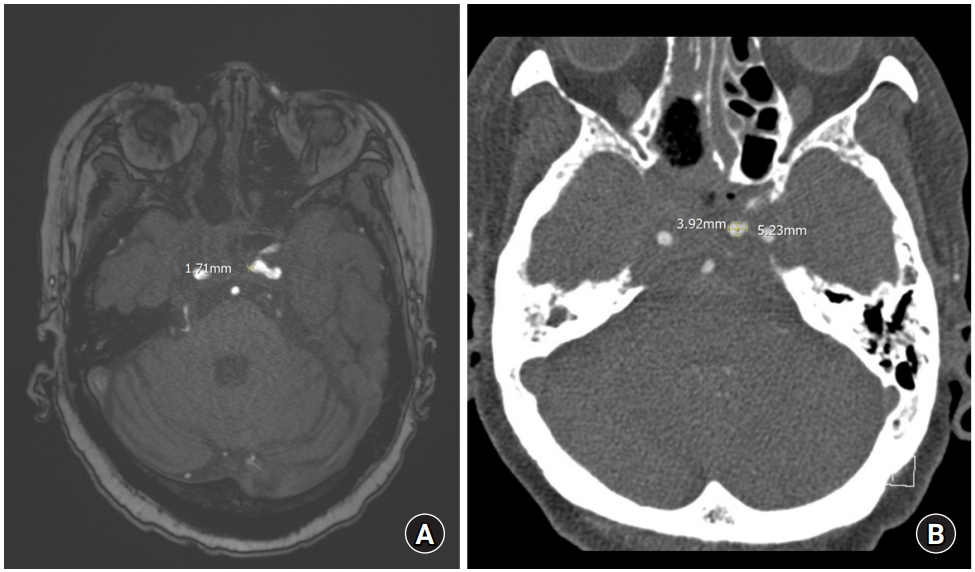
(A) Magnetic resonance angiography image. An early aneurysm is suspected since a long diameter of about 1.7 mm is observed on the left cavernous internal carotid artery. (B) Computed tomography angiography image. Despite the short interval, the aneurysm of the left cavernous internal carotid artery previously observed showed a significant increase in size up to 5.2 mm in the long diameter.

Digital subtraction angiography image. On left internal carotid artery angiogram, the aneurysm was observed with lobulated configuration, arising from the left cavernous internal carotid artery, showing inferior direction.
Endovascular treatment
At the time of endovascular intervention, because the size of the previously identified lesion was increased to a long diameter of 20 mm, coiling was not feasible, and cross-filling through the anterior communicating artery was confirmed via a left common carotid artery (CCA) compressed right ICA angiogram. Consequently, endovascular ICA trapping was performed (Fig. 4). Subsequent angiogram confirmed a contrast leakage in the dome part of the aneurysm sac. Thereafter, 13 coils were deployed from the distal cavernous ICA to the petrocavernous junction using Target 360 Ultra 5 mm × 15 mm. Nine pushable coils (Mnester) were inserted into the distal, neck, and proximal portions of the aneurysm and petrous ICA. A subsequent left CCA angiogram showed that the antegrade of left proximal ICA was obliterated, and that the distal ICA was contrasted through external carotid artery-ophthalmic anastomosis. The cross-filling through the anterior communicating artery had no significant difference from that observed in the ICA angiogram performed before the procedure.
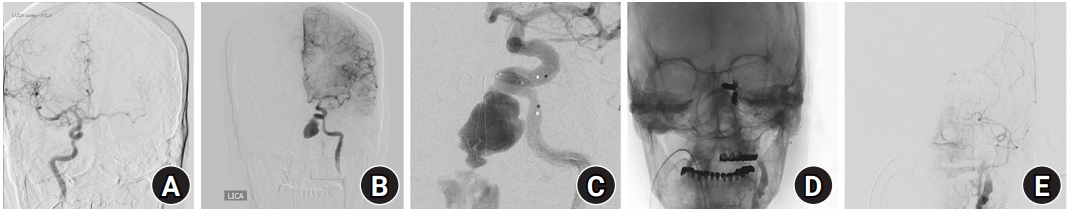
Digital subtraction angiography image. (A) Cross-filling through the anterior communicating artery on the internal carotid artery angiogram was checked. (B) The left cavernous internal carotid artery pseudoaneurysm increased to a long diameter of 20 mm. (C) Contrast leakage from the dome portion of the aneurysmal sac after stent deployment. (D, E) Complete obliteration of the left petrocavernous internal carotid artery is shown.
Postoperative course
The patient received care in the intensive care unit after intervention. During the nine days of intensive care, no additional neurological deterioration was identified; subsequently, the patient was transferred to a general ward without any special events. The patient complained of intermittent headaches but recovered gradually. The patient was stably discharged eight days later.
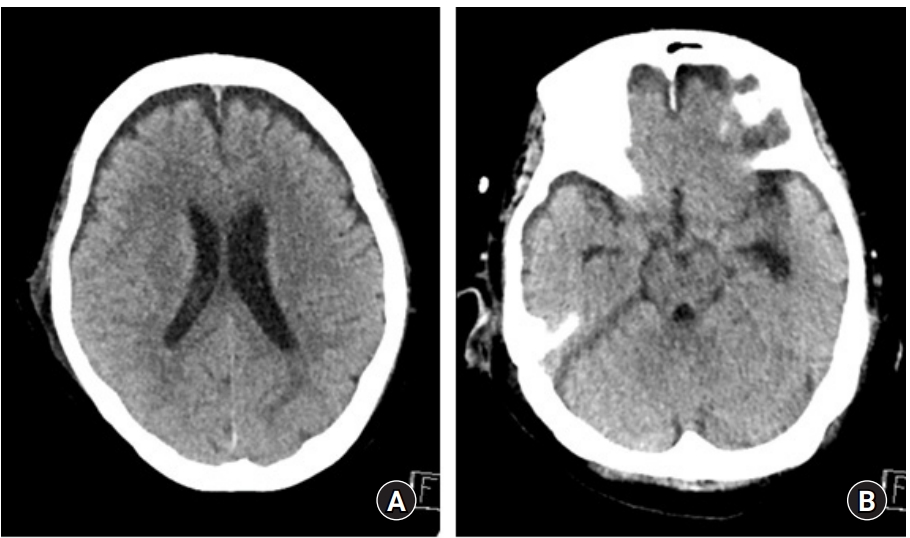
Eight days after discharge, the patient visited our emergency center with altered mental status, symptoms of posterior nasal drip and headache. Neurological examination showed a Glasgow coma scale score of 9. Otolaryngologic examinations revealed CSF leakage and erosive skull lesions. Brain CT showed pneumocephalus (Fig. 5). Thus, intracranial hypotension due to CSF leakage was diagnosed which was thought to be the cause of the patient’s mental status deterioration. Therefore, conservative treatment involving lumbar drainage was performed. After lumbar drainage, CSF leakage and pneumocephalus disappeared and the patient was slightly improved (Fig. 6); however, due to delirium and poor general condition, no significant improvement in consciousness was exhibited. Moreover, aspiration pneumonia occurred; therefore, the patient was transferred to hospice after tracheal intubation.
DISCUSSION
Most nasopharyngeal cancers are treated with radiation therapy, and complications after radiation therapy are often managed by oncologists or otolaryngologists1-3). However, as seen in this case, fatal neurosurgical complications may also occur. In addition, there have been reports of other neurosurgical complications such as brain abscess, CSF leakage, carotid artery stenosis, aneurysm, temporal lobe necrosis, and clinical hypopituitarism1-3,5-7).
Moreover, there have been cases in which vasculopathies that occurred after such radiation therapy were treated with neurosurgical endovascular procedures4,5,7-10). Therefore, neurosurgeons need to be alert regarding the evaluation and treatment of complications of nasopharyngeal cancer.
The anatomical position of the nasopharynx is close to the skull base, which is thought to be a risk factor for tumor invasion or radiation therapy to cause neurosurgical complications. This assumption adds credibility that CSF leakage is reported not only in nasopharyngeal cancers that have undergone radiation treatment but also in other nasopharyngeal tumors where radiation treatment is not administered11).
Treatment methods for pseudoaneurysms are generally categorized into surgical and vascular.
Surgical treatments of pseudoaneurysms that are traditionally used include ICA ligation and trapping clipping with or without an extracranial-intracranial bypass12). However, unlike a true aneurysm, the vascular wall of a pseudoaneurysm is very thin and has a high risk of rupture13). Moreover, because of the development of increasingly advanced intravascular procedures, many cases are being managed with endovascular treatments.
Endovascular stent insertion with or without endosaccular occlusion is an effective endovascular treatment14). Endosaccular occlusion alone has been reported in cases where the coil is exposed due to osteonecrosis and degeneration of surrounding tissues after radiation therapy, and accordingly, the use of an endosaccular stent is a safer method4). Other studies point out the limitations of treating aneurysms using stents alone in that it leads to thrombosis of the aneurysm; however, since the placement of stents helps in complete coiling, it would be better to combine coiling and stent insertion14).
Endovascular trapping is an effective treatment provided there is sufficient collateral blood flow4). This can be done by using detachable balloons or microcoils. Treatment with microcoils can achieve fast and permanent obliteration; however, the risk of a distal embolic event due to thrombogenesis has been reported4). Heparinization and temporary proximal flow arrest during the procedure have been reported as ways to lower the risk of these problems15).
In our case, coiling was not chosen due to the increased size of the aneurysm in a short time; subsequently, ICA trapping was planned. Since contrast leakage was confirmed after stent deployment, ICA flow was stopped immediately; hence, microcoils were employed. Likewise, heparinization was not implemented because its benefits were less compared to the risks involved. Usually trapping is performed using only microcoils without a stent; however, since the neck of the aneurysm was wide in this case, stent deployment was performed to protect the aneurysm sac by preventing coil herniation.
Applying a vascularized flap is good for the treatment of CSF leakage16). Although the method of implementing craniotomy is common, Daniel et al. argued that repairing CSF leakage with an endonasal endoscopic approach was less invasive and safe17). Although we had planned CSF leakage repair using such a vascularized flap, surgery was not feasible as the patient had terminal cancer. Therefore, lumbar drainage was performed, and conservative treatment was undertaken. However, aspiration pneumonia eventually occurred, and the general condition deteriorated.
CONCLUSIONS
Complications in nasopharyngeal cancer patients require neurosurgical attention and immediate acute intensive care and first aid if necessary.
Notes
Conflict of interest
No potential conflict of interest relevant to this article was reported.