Negative-Pressure Hydrocephalus Treated with a Modified Shunt System: A Case Report
Article information
Abstract
A negative-pressure hydrocephalus (NePH) is a rare type of hydrocephalus that is symptomatic despite the negative intracranial pressure (ICP). Because of the shortcomings of the existing shunt system that could not produce the necessary gradient for an effective cerebrospinal fluid (CSF) drainage in NePH patients, a refined method for CSF drainage is needed. We combined the traditional low-pressure valve with a distal catheter, which has no end hole and only has side slits, to prevent the CSF backflow and facilitate the siphon effect. Simultaneously, the active pumping of the shunt reservoir was also conducted to remove the excess CSF from the ventricular system. The treatment of NePH requires an appropriate reduction of excessive CSF until the CSF dynamics and brain compliance are restored. We suggest the use of this modified shunt system for NePH treatment, as it is easily modifiable and has sufficient effects.
INTRODUCTION
A negative-pressure hydrocephalus (NePH) is a rare type of hydrocephalus characterized by the presence of negative intracranial pressure (ICP) and ventriculomegaly. Despite the low or negative ICP, patients with NePH often develop symptoms consistent with high ICP, and they frequently deteriorate. Several hypotheses have been proposed to demonstrate the underlying pathophysiology in NePH, but the exact mechanism still remains unclear. Multiple treatment options have been suggested, including the identification and repair of cerebrospinal fluid (CSF) leak, subatmospheric external ventricular drains (EVD), neck wrapping, or placement of CSF shunts. In this study, we present a case of a NePH patient refractory to the standard CSF shunt procedure and successfully treated with a modified ventriculoperitoneal shunt system.
CASE REPORT
A 55-year-old man with a history of anaplastic oligodendroglioma, for which the patient underwent surgical tumor resections followed by radiotherapy and chemotherapy, presented with a local tumor recurrence at the left medial frontal lobe. He underwent gross total resection of the tumor via trans-ventricular approach through the previous surgical defect (Fig. 1A, B). After the surgery, the patient had no postoperative complications, except for mild cognitive impairment, and he was discharged after a week of routine hospital care.
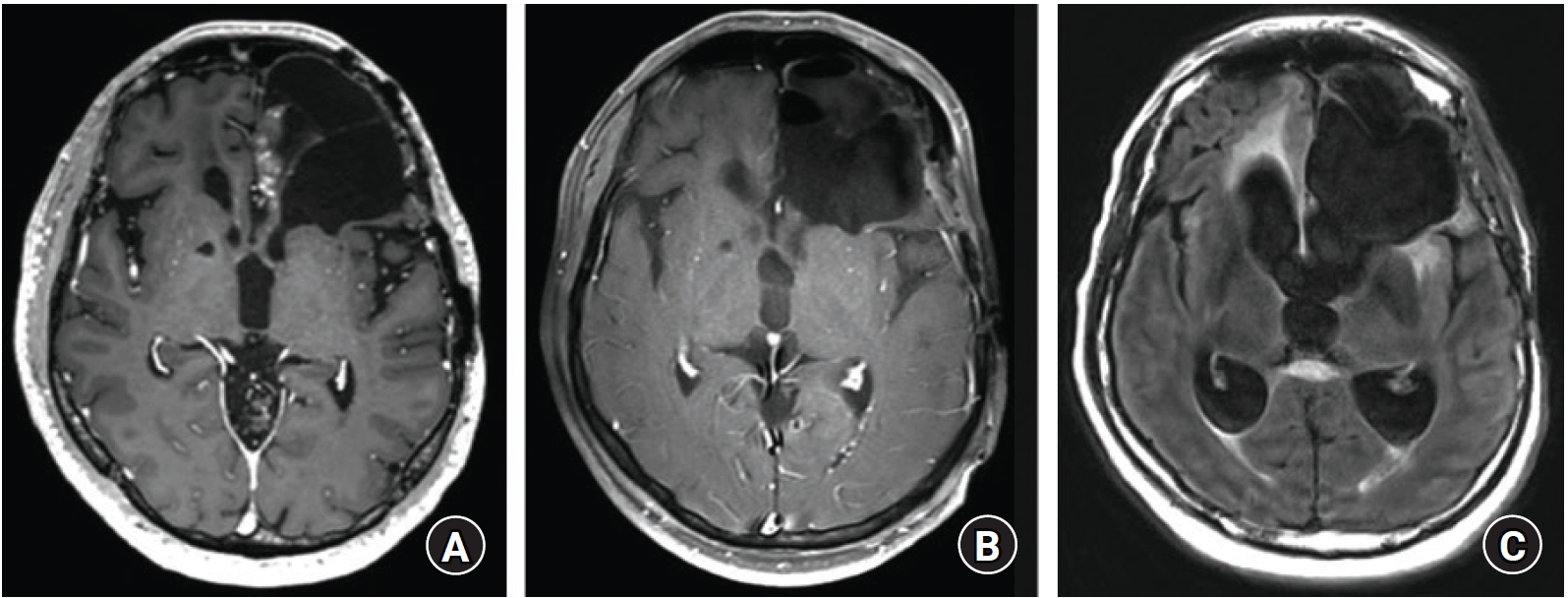
(A) The preoperative magnetic resonance imaging (MRI) shows an enhancing mass in the left medial frontal lobe. (B) The postoperative MRI shows the gross total resection of the tumor. (C) Two months later, the follow-up MRI shows the marked interval progression of hydrocephalus.
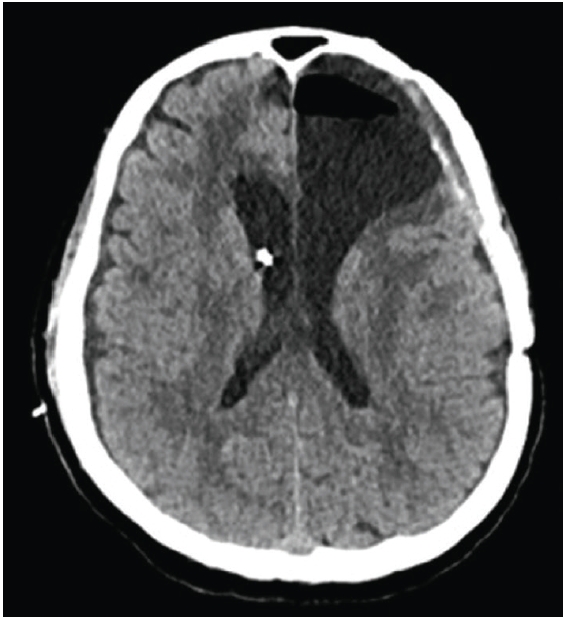
After two months, the patient showed progressive gait disturbance and cognitive impairment, and his follow-up magnetic resonance imaging (MRI) revealed significant hydrocephalus (Fig. 1C). Ventriculoperitoneal shunt (VPS) with a programmable valve (Miethke ProGAV Programmable Shunt System; Aesculap, Inc.) was implanted and programmed at the lowest pressure setting (0 cmH2O) based on low opening pressure (Fig. 2A).
(A) Immediate postoperative computer tomography (CT) scan after ventriculoperitoneal shunt implantation. (B) Two days later, the CT scan shows the increased ventricle size.
The patient temporarily improved; however, after two days, he worsened again. He became drowsy and could not communicate verbally. His head computed tomography (CT) scan revealed the increased size of ventricles, suggesting that the shunt was not working properly (Fig. 2B). After tapping the shunt reservoir and draining 50 cc of CSF, his symptoms improved quickly. The VPS was removed, and EVD was inserted instead. The EVD was lowered to −10 to −5 cmH2O to achieve CSF drainage, and the patient responded to subatmospheric CSF drainage.
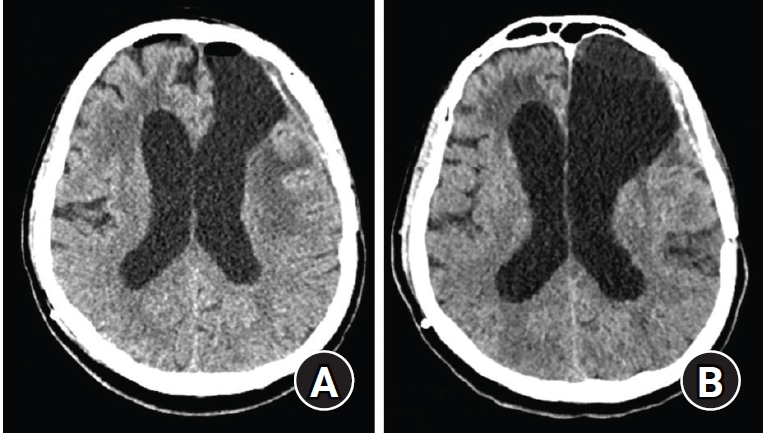
The removal of CSF by >200 mL per day significantly improved his neurological status, and the CT scan finding showed the decreased size of ventricle (Fig. 3). To restore the brain compliance, the subatmospheric CSF drainage was kept for more than two weeks. However, his ICP was constantly negative, and we tried to wean him several times from CSF drainage but failed. The patient remained incapacitated in supine and head-flat position and unable to be discharged from the intensive care unit. After three weeks, another ventriculoperitoneal shunting was tried. Considering the limitations of the previous “traditional” shunt system, a modified shunt system was devised. The shunt system consisted of 1) a 5M (10.5 Fr) EVD catheter as the proximal catheter, which had a larger diameter than the usual proximal VPS catheter that could prevent the occlusion; 2) a non-programmable Medtronic valve (CSF-flow control valve, low-pressure type, valve opening pressure of 3 cmH2O), which had the largest dome of reservoir pump in our center, for an active pumping to reduce a larger CSF volume; and 3) a distal peritoneal catheter tied at the end tip, which only had side slits and no open end to prevent reflux from an insufficient pressure gradient and facilitate the siphon effect. The ventricular (proximal) catheter was inserted through the right Frazier’s point.

After the external ventricular drains insertion, followed by a subatmospheric cerebrospinal fluid drainage for two days, the computer tomography scan shows the improvement of hydrocephalus.
Postoperatively, we instructed the nursing staff to actively pump the reservoirs, and the patient showed remarkable clinical improvement. The patient had the best neurological condition when about 100 mL of CSF was drained per day. A single compression of the pump removes about 0.5 mL of CSF (based on the reservoir volume); an average of 12 pumps/h for 16 h per day, excluding the sleeping time, was recommended. The follow-up CT scan findings were consistent with the clinical improvement (Fig. 4B), and the patient was able to ambulate on his own. After being discharged to the general ward, the patient and his caregiver were educated with proper instructions for the active pump. Eventually, he became independent in performing simple activities of daily living (mRS 2), and he was discharged after a week of shunting.
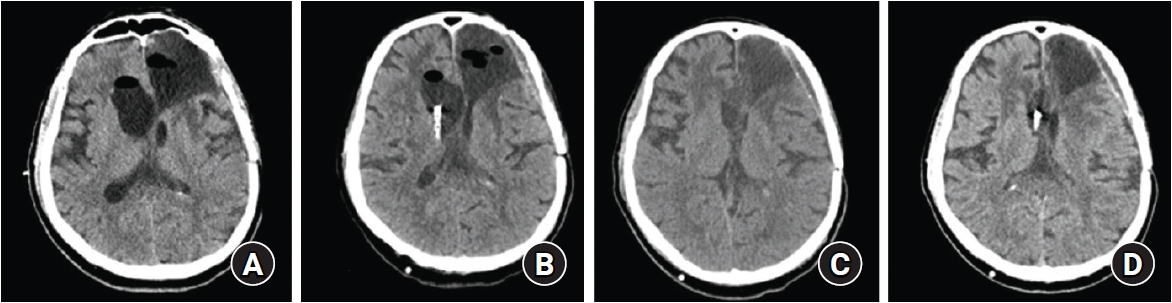
(A) Computer tomography (CT) finding after a month of external, subatmospheric cerebrospinal fluid drainage. (B) A proximal shunt catheter was inserted through the right Frazier’s point. (C) CT scan after the clinical deterioration from an excessive pumping, showing a marked decrease in ventricle size. (D) Despite not being pumped for a week, the ventricle size did not change.
After a month, he returned to our emergency room with altered mental status, and the CT scan revealed a slit ventricle, suggesting over-drainage (Fig. 4C). Based on history taking, it was found that his caregiver did an excessive reservoir pumping, leading to the development of slit ventricle syndrome. As the patient got worse, the caregiver performed even more pumping. By being in a supine position with his head flat and inhibiting the reservoir pumping, the patient recovered from being drowsy. After a week without pumping, he did not experience another neurological deterioration, and the ventricle size was consistent in the follow-up CT scan (Fig. 4D). He was free from the active pumping and discharged for regular follow-ups. He remained asymptomatic, and six months later, his follow-up MRI findings showed no interval changes in the ventricle sizes (Fig. 5).
DISCUSSION
A hydrocephalic state without an elevated ICP has been widely described after the introduction of a clinical condition known as normal pressure hydrocephalus (NPH)1). Recently, symptomatic hydrocephalus with low or negative ICP has been attempted to be identified as a subgroup of hydrocephalus, described as low-pressure hydrocephalus (LPH), very low-pressure hydrocephalus, or NePH. Previous studies have described multiple cases of these rare, complex clinical conditions and proposed several pathogeneses. Fillipidis et al. proposed a theory that emphasized the role of transmantle pressure in developing NePH or LPH, that the blockage of the CSF pathways between the ventricles and cortical subarachnoid space with CSF leak may establish a pressure gradient, leading to ventriculomegaly with negative ICP2). Moreover, they proposed another theory suggesting the role of brain turgor, which was demonstrated as the alteration in brain compliance may lead to ventriculomegaly when the negative ICP persists, as previously described by Pang and Altschuler3).
Recognizing the complex pathophysiology of NePH, several treatment options different from the classical “CSF diversion” were attempted. Identifying and repairing the CSF leak has been advocated to eliminate the transmantle pressure2). In addition, a titrated subatmospheric EVD has also been applied to reestablish the CSF communication and change the brain viscoelasticity4,5).
However, in certain cases, the mentioned treatment strategies may be limited or insufficient. EVD should be prolonged, as enough time is needed for the brain to regain its viscoelasticity, but it becomes more susceptible to infection. Moreover, a prolonged external drainage is inconvenient and keeps the patients incapacitated. In a previous case report, Kalani et al. attempted the active pumping negative-pressure shunt system in patients who were refractory to routine treatment options6). With an active pumping shunt, which had a reservoir system on the patient’s sternum, actively compressing the reservoir pump allowed the patients to improve.
In this case, the patient also underwent subatmospheric external CSF drain for several days. Although he had significant clinical improvement during this period, the prolonged EVD resulted in immobilization and incapacitation. His ICP was constantly negative, and weaning off EVD failed repeatedly. A conventional shunt system already failed once in the patient, for it could not establish a low-pressure gradient enough to remove the CSF from his ventricular system. Thus, a modified shunt system was tried by manipulating the existing apparatus for practical reasons, and it worked properly. By this maneuver, the patient recovered from his hydrocephalic symptoms and did not require further external drainage.
Although he suffered from slit ventricle syndrome after the immoderate pumping, eventually, he no longer needed an active pumping without the recurrence of hydrocephalus. Thus, this modified shunt system can be considered successful in “resetting” the brain parenchymal compliance, in accordance with the theory of elastic hysteresis provided by Lesniak et al.7) In NePH patients with constantly negative ICP that could not be restored to equilibrium after a prolonged EVD, a shunt system with an active pumping method can be helpful.
CONCLUSION
In this case, we have described a successful treatment for a NePH patient using a modified ventriculoperitoneal shunt system. However, the pathophysiology of NePH still remains unclear, and more studies are required to provide the evidence to propose treatment strategies.
Notes
Conflict of interest
No potential conflict of interest relevant to this article was reported.
Acknowledgements
None