Anaesthetic Management of Systemic Injuries Requiring Surgical Intervention in Patients With Spinal Shock
Article information
Abstract
Spinal cord injury is a devastating event, and patients who have suffered a spinal cord injury may present to trauma emergency services in spinal shock. This subset of patients is likely to have concomitant systemic injuries, which may often require surgical interventions. All trauma patients should get sufficient pre-hospital care, including adequate measures to immobilize the spine. Because multiple organ systems are involved in these patients with spinal shock, anesthetic management for systemic injuries requiring surgery is challenging and necessitates a thorough understanding of the pathophysiology. The main goals of perioperative management are to provide oxygenation and maintain spinal cord perfusion to prevent secondary spinal cord injury while providing supportive care. Airway management invites a trained anesthesiologist to secure the airway while maintaining cervical spine immobilization. In this article, we review anesthetic considerations in managing spinal cord injury patients with systemic injuries in spinal shock requiring surgical interventions.
INTRODUCTION
During the year 1840, Hall coined the phrase "spinal shock"1). Sherrington went on to describe this as a transient cessation of reflexes below the level of spinal cord damage2). After an acute onset of spinal cord injury (SCI), there may be a sudden loss of reflexes and muscle tone below the level of injury. This condition is known as “spinal shock,” which can develop with SCI at any level3). Spinal shock should be distinguished from spinal neurogenic shock. The latter describes the symptoms of decreased systemic vascular resistance with hypotension, bradycardia, and hypothermia that develop following acute cervical and upper thoracic spinal cord damage when the regulation of the sympathetic nervous system on hemodynamic is disrupted. In this article, we want to review the anesthetic management of systemic injuries requiring surgical intervention in patients with spinal shock.
MANUSCRIPT BODY
Etiology
Spinal cord injury (SCI) can occur as a result of a variety of traumatic or non-traumatic causes. Motor vehicle collisions are the most common traumatic cause of spinal cord injuries. Falls, violence, and sports-related injuries constitute other traumatic causes. Neoplastic, vascular, infectious, and hereditary-degenerative diseases are the common non-traumatic causes4). SCIs commonly affect the cervical spinal column, followed by the thoracolumbar junction4).
Pathophysiology
The physiological function of the spinal cord will be temporarily interrupted in patients who have suffered a SCI and subsequent spinal shock. Mechanical force from bony fragments, joint dislocations, ligamentous tears, and herniated intervertebral discs usually results in cord compression and contusion during SCI, contributing to primary injury5). A critical fall in spinal cord perfusion due to vasospasm, injury to intramedullary arteries, and subsequent hemorrhage into gray matter causes secondary injury. Inflammation, ischemia, hypoxemia, hyperthermia, edema, and mediators from post-ischemic injury, free radical production, lipid peroxidation, ionic derangements and apoptosis of neurons are all associated with secondary damage. This underlying pathologic process causes further cord edema, which peaks 4-6 days following the damage6,7). The spinal cord below the level of the lesion is isolated from higher centers, and classic flaccid paralysis ensues. Early administration of high-dose methylprednisolone, as demonstrated by the National Acute Spinal Cord Injury Study (NASCIS) trials, is followed in many specialized spine centers to improve long-term neurological outcomes.
Diagnosis
Until otherwise demonstrated, the cervical spine is first assumed to be unstable in all trauma victims. The main objective of imaging is to quickly and precisely identify injuries to the spine that could endanger neural tissue. All trauma patients with risk factors for spinal cord or spine injury should have imaging done. These patients include those who have neck pain or tenderness, neurologic deficits, an impaired level of consciousness, intoxication, or painful distracting injuries. When all of these clinical risk factors are absent, a cervical spine injury can be ruled out with a high degree of confidence, according to several studies8-10).
The antero-posterior, lateral, and odontoid views are included in standard cervical spine radiographs. Lateral films should be thoroughly examined for anomalies in vertebral alignment, bony structure, intervertebral space, and soft tissue thickening. A higher level of accuracy might be obtained by helical computed tomography (CT), which enables sagittal and three-dimensional reconstruction11). Magnetic resonance imaging (MRI) is the modality of choice for identifying acute cord injuries12). MRI also detects ligamentous injury, spinal cord edema and hemorrhage, and other non-osseous changes that may go undetected by other methods. SCI without radiographic abnormalities (SCIWORA) is the term used to describe patients with cord-induced deficits who do not have any spinal abnormality or injury that can be seen on plain radiographs or CT. It could happen in 2.8–3.8% of all spinal injuries13,14). Physical examinations should pay close attention to non-spinal cord injuries, which affect the head, chest, or abdomen most frequently in 20% to 60% of patients with SCI5). Abdominal ultrasound, CT, or diagnostic peritoneal lavage might help in ruling out intra-abdominal bleeding.
Anesthetic management
These systemic injuries may sometimes require simultaneous surgical management. Immediate resuscitation, stabilization of vital organ function, and prevention of secondary injury are the main components of the management of patients with SCI. The common systemic injuries that require emergency surgery may include, but are not limited to, any solid organ injuries, either in the thorax (lung or heart injuries leading to hemothorax) or abdomen (liver lacerations, spleen lacerations, intestinal perforations leading to hemoperitoneum, peritonitis), major vascular injuries, major pelvic injuries, and traumatic amputations of limbs.
Airway management
The place of airway management (trauma unit or operating room) is dictated by the condition of the patient. It is necessary to check the patency of the airway and, if required, support with a jaw thrust rather than a chin lift, as the former is associated with less cervical spine displacement. If the airway is patent, the patient should be given a trauma mask with high-flow oxygen. If not, it is best to intubate the trachea as soon as possible to maximize oxygen delivery and minimize hypoxic secondary injury to the injured spinal cord. Potential poor laryngoscopic view due to blood, debris, or distorted anatomy in concomitant facial trauma, immobilization of the cervical spine, and rapid sequence induction might contribute to the possibility of a difficult intubation15).
Although cricoid pressure has been thought to dislocate the injured spine, a cadaver study using a lateral cervical spine x-ray revealed minimal spine movement when it is applied together with manual inline stabilization (MILS) 16). MILS reduces but does not completely prevent cervical spine movement during laryngoscopy (Fig. 1) 17). In patients under anesthesia, MILS reduces extension by 50% between the occipital bone and C1 and between C1 and C218). However, while using MILS, the laryngeal view during direct laryngoscopy may deteriorate, which could increase the chance of a failed tracheal intubation19). Additionally, it also increases the maximum force transferred during direct laryngoscopy20). During direct laryngoscopic intubation, the gum elastic bougie is a key adjuvant to prevent displacement of the fractured spine21). It permits higher-grade laryngoscopy views of the vocal cords, thus reducing the forces transmitted to the spine. We have never encountered a failed intubation due to MILS. Despite higher grades of laryngoscopic views with MILS, it is routine practice in our institute to use either anterior laryngeal pressure or gum elastic bougie to facilitate tracheal intubation.
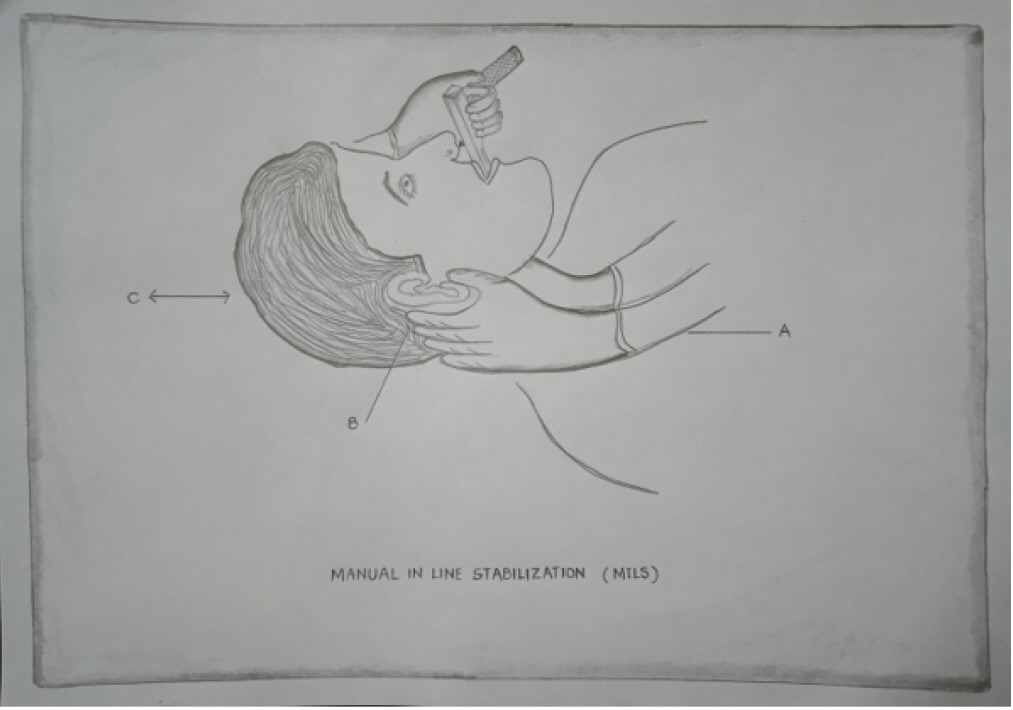
Line diagram showing application of manual in-line stabilization (MILS) during direct laryngoscopy. A: rest forearms on the patient's chest; B: grasp the patient's mastoid processes and occiput with both hands; C: maintain neck alignment to limit as much movement of the head and cervical spine as possible without traction or counter-traction during laryngoscopy.
In patients with cervical spine immobilization, video laryngoscopes reduce intubation difficulty. In a cadaveric study comparing three different types of video laryngoscopes (GlideScope; C-MAC D-Blade; and McGrath MAC X-blade) with Macintosh blade, the authors concluded that all of the video laryngoscopes induced similar amounts of displacement at all cervical spine segments (C1-C6), which was much less than what was observed with the Macintosh laryngoscope22). There was no difference between the change in space available for cord with a Macintosh blade and the Airtraq video laryngoscope in cadaveric models of C3/C4 injury and type-2 odontoid peg fracture23-25). However, the King Vision aBlade did result in a lower change in space available for cord compared to a Macintosh blade in a cadaveric model of atlanto-occipital instability26).
The choice of intubation strategy is determined by specific airway characteristics, the level of the patient's cooperation, and the operator’s expertise27). The latter two are crucial for the success of the awake intubation. Elective airway management facilitates awake fiberoptic intubation and allows for unhurried airway topicalization. Flexible bronchoscope-guided tracheal intubation is regarded as the gold standard for patients with cervical spine injuries. This is partly because it could be done while the patient is conscious, allowing for a neurological evaluation following tracheal intubation and before general anesthesia is induced19). It is also a desirable choice as immobilizing devices do not need to be removed, and displacement of the spine is minimal in expert hands. However, unwanted neck movement may occur if the local airway anesthesia is ineffective. No single strategy of airway management has been proven to be superior to others. When MILS is ensured during intubation, there is no evidence to show an association between the technique of intubation and neurological decline. As a result, the operator should be able to secure the airway using the approach that they are familiar with without worrying about damaging the cord. Epistaxis, laryngospasm, and oesophageal intubation are some of the complications associated with blind nasal intubation. Less cervical spine movement is produced during intubating LMA insertion than during direct laryngoscopy28).
Direct laryngoscopy combined with MILS is an acceptable and safe procedure. Most centers recommend rapid sequence induction with thiopentone and succinylcholine. Succinylcholine can be used safely until 48 h post-injury, after which a hyperkalemic response due to denervation hypersensitivity should be anticipated29). Gentle mask ventilation can be considered if the benefit of hypoxemia correction outweighs the risk of aspiration, as proper preoxygenation in the setting of emergency intubations is not always possible. The attending anesthesiologist has the option to select the anesthetic medications, and this decision is frequently influenced by the patient's clinical condition, drug availability, and institutional policies. MILS application requires additional, trained anesthesia personnel. The back of the rigid cervical collar is left in place to prevent movement of the injured cervical spine by cricoid pressure. If not, a bimanual approach with one hand under the neck, counteracting the downward pressure on the cricoid cartilage, should be used.
Monitoring
Along with standard American Society of Anesthesiologists monitors, invasive blood pressure monitoring is almost always required. It aids in beat-to-beat monitoring and facilitates sampling for arterial blood gas analysis. It is always useful to have central venous access for guiding fluid resuscitation and for the administration of a possible vasopressor or inotropic infusion. Monitoring systemic vascular resistance is advised since it is lower in patients with spinal shock. A pulmonary artery catheter or trans-oesophageal doppler may be needed in more complex cases. Ascending sensory pathways in the posterior columns are evaluated by somatosensory evoked potentials (SSEP), whereas descending corticospinal tracts are assessed by motor evoked potentials (MEP)30,31). Multimodal intraoperative neuromonitoring (MEP and SSEP) may alert the operating team to any deterioration in spinal cord function and provide an opportunity to address the contributing factors during surgery. These factors include the patient’s position (such as neck or shoulder position), hypotension, hypothermia, and surgical aspects of the operative procedure.
Role of immobilization
The main aim of immobilization is to prevent or restrict further secondary neurologic injury in patients with unstable spines following SCI. Because spinal injury can occur at multiple non-contiguous levels, the entire spine should be immobilized until the appropriate physical examination and imaging have ruled out injury. The proper technique of immobilization includes placement of a rigid cervical collar of suitable size, sandbags on either side of the head, attachment of adhesive tape across the forehead to each side of the trolley, transport of the patient on a hard spine board, and log-rolling wherever indicated, maintaining vertebral column alignment. However, immobilization is not without complications. More than half of the patients may develop pain, pressure sores, or diminished chest wall motion. The risk of airway compromise, difficult intubation, aspiration, and raised intracranial pressure may also increase with neck immobilization32).
Role of steroids
In cases of acute SCI, methyl prednisolone reduces the release of interleukins, prostaglandins, and thromboxanes through its anti-inflammatory and cell membrane stabilization properties. It helps in reducing spinal cord edema and increasing perfusion to the injured cord. Methyl prednisolone (30 mg/kg IV followed by 5.4 mg/kg/h for 23 hours) and naloxone (5.4 mg/kg IV followed by 4.0 mg/kg/h for 23 hours) were compared in the NASCIS-II trail33). Motor function significantly improved when methylprednisolone was given within 8 hours of the injury at both 6 months and 1 year. However, the improvement was not functionally significant. In the NASCIS-III trial, further benefit was achieved by extending the administration of methyl prednisolone up to 48 h in patients who presented between 3 and 8 h after SCI. Adverse effects of steroid administration like increased blood glucose levels, myelopathy, wound infections, and gastrointestinal bleeding should be managed.
Intra operative considerations
General anesthesia is nearly always required for these surgical patients with SCI and spinal shock. Slow anesthetic induction is recommended as they are more sensitive to the hypotensive effects of anesthetic drugs on the background of relative hypovolemia and reduced sympathetic output. Additionally, positive pressure ventilation will also put them at risk of hypotension. Intraoperative fluid management for these patients with systemic injuries requiring surgical intervention can be challenging. The ideal fluid therapy for these patients remains unclear. However, it is better to avoid hypotonic crystalloids like D5W and 0.45% normal saline, which may worsen spinal cord edema. Isotonic crystalloid fluid boluses should be used to treat profound hypotension. However, repetitive fluid boluses are not advised if hypotension is found to be caused by spinal shock (as opposed to volume loss from hemorrhage caused by other injuries), and the patient should be given inotropes or vasopressors to maintain mean arterial blood pressure.
An agent having inotropic, chronotropic, and vasoconstrictive properties is required for injuries at the cervical and upper thoracic levels. Dopamine, norepinephrine, or epinephrine are substances that meet these criteria34). Due to its effect on vasodilation and potential for reflex bradycardia, dobutamine only has a limited role as an inotropic drug in this subset of patients34,35). Norepinephrine was found to be better than dopamine or phenylephrine at improving spinal cord perfusion with fewer side effects36). Strategies like the use of antifibrinolytic agents and recombinant factor VIIa have been shown to reduce intraoperative blood loss in these patients undergoing spine surgeries4). Emergency hemorrhage panel, which includes hematocrit, prothrombin time, fibrinogen and platelet count, has been developed with quick results that could guide the transfusion decisions in these patients37).
In the prone position, pressure points need to be meticulously protected. Overzealous fluid administration in the prone position may be associated with airway edema, cardiac failure, electrolyte abnormalities and prolonged intensive care unit stay38). Changes in position may have major hemodynamic effects; abrupt adoption of the head-up position may cause severe hypotension through venous pooling of blood, whereas the head-down position may result in cardiac failure. A thoracotomy may be required for some injuries, and in that case, we may need to provide one-lung ventilation to facilitate surgery.
MEP monitoring necessitates total intravenous anesthesia (TIVA), which is the main impact of electrophysiologic monitoring on anesthesia practice. Inhalational anesthetics at a dose of less than 1 minimum alveolar concentration (MAC) can be used when SSEPs are being monitored. During MEP monitoring, it is recommended to avoid volatile anesthetics and nitrous oxide and use TIVA without muscle relaxation39). Starting at low concentrations, volatile anesthetics elicit a dose-dependent reduction in MEP signal amplitude. Opioids have no effect on evoked potential monitoring. Dexmedetomidine has been commonly used to reduce the dosage of propofol during the administration of TIVA40,41). The MAC of volatile inhalational anesthetics is unaffected by decerebration or cervical cord transection, suggesting that the site of action for the anesthetic effects is at the cord level for noxious stimuli under general anesthesia42). Thiopental may have a neuroprotective impact on the spinal cord43).
Hypotensive anesthesia as a means of blood conservation is not a choice for patients with pre-existing SCI. Avoiding hypotension or hypovolemia is advised, and the mean arterial blood pressure should be kept above 80 to 85 mm Hg. This might necessitate the administration of blood and/or vasopressors. Vagal stimulation during tracheal suctioning might precipitate bradycardia and sometimes asystole. As a result, having vagolytic medications on hand and performing adequate oxygenation before attempting tracheal suction in these patients is critical. It is necessary to take the usual precautions to protect all pressure points during positioning. SCI worsens when the neck is hyperextended during positioning. Heated humidifiers and forced air warming devices will help in preventing hypothermia. Deepening the plane of anesthesia during surgery can be an effective way to address complications like autonomic dysreflexia and muscle spasms. Severe factors like type of surgery, duration of surgery, intraoperative positioning, intraoperative complications, hemodynamic stability, ease of intubation, etc. should be considered before planning extubation at the conclusion of surgery.
SPECIFIC CONSIDERATIONS
Respiratory considerations
In patients with cervical SCI, significant changes in respiratory mechanics, breathing patterns, ventilatory control, and bronchial reactivity can be observed44). Reduced pulmonary and chest wall compliance increases the work of breathing45). Rapid, shallow breathing with a restrictive pattern of pulmonary function testing is not uncommon46). The degree of respiratory support required in this subset of patients is determined by the level of SCI. Complete SCI above the level of C3 results in apneic respiratory arrest and death in the absence of prompt ventilatory support. Various levels of respiratory failure are linked to a C3-C5 injury. Less severe ventilatory impairment is associated with injuries below the C5 vertebra, but patients are still at risk for pulmonary complications.
Cardiovascular considerations
Systemic hypotension and reduced spinal cord perfusion pressure are typical complications of traumatic SCI47). They subsequently exacerbate secondary neurologic damage. Interruption of cardiac accelerator fibres (T1-T4) is proposed as the cause of bradycardia and decreased myocardial contractility. This cardiovascular depression in some of these patients may be preceded by a brief episode of acute hypertension that is assumed to be caused by the enormous, simultaneous discharge of sympathetic neurons. The subendocardial myocardial injury and neurogenic pulmonary edema that have been noted after SCI and other types of central nervous system injury may be caused by this hyperadrenergic response48). High SCIs can occasionally be accompanied by ECG abnormalities, including signs of subendocardial ischaemia and arrhythmias. In the presence of myocardial depression, which is common following SCI, pulmonary edema develops as a result of overzealous fluid administration to correct spinal shock.
Deep venous thrombosis
Venous thromboembolism is a specific risk for people with spinal injuries. For those who do not get prophylaxis, the incidence of deep venous thrombosis (DVT) ranges from 39% to 100%47). In patients with SCI, low molecular weight heparin or low-dose unfractionated heparin, combined with nonpharmacologic devices (like pneumatic compression devices and graduated compression stockings), was found to be effective for antithrombotic prophylaxis. Inferior vena cava filters should not be used as the primary thromboprophylaxis in these patients49).
Gastrointestinal considerations
In patients with acute SCI, the risk of stress ulcers and upper gastrointestinal bleeds is increased. This is exacerbated in patients who are on mechanical ventilation and receiving high-dose steroid treatment. The risk of aspiration and subsequent postoperative pulmonary complications is also increased in this subset of the population because of gastric ileus and delayed gastric emptying. So, placing a nasogastric tube is necessary.
Neuropsychiatric considerations
A spinal cord injury is a catastrophic event and these patients may have significant psychological discomfort. Depression, anxiety disorders, substance-related disorders, and suicidal tendencies are mentioned in the literature among patients with SCI50,51). A humane and empathetic approach and good communication are crucial.
Autonomic dysreflexia
The most significant and pertinent SCI consequence for anesthesiologists is Autonomic dysreflexia (ADR). Symptoms can appear weeks or years after the initial injury. It is a clinical emergency with a cluster of symptoms characterized by a severe, disrupted autonomic response to particular stimuli below the level of the spinal cord lesion52). Distension of hollow viscera (bladder, bowel, uterus, and gallbladder), cutaneous stimulation, and surgical procedures often involving pelvic organs or the lower extremities are the common triggering events30,52). It occurs after spinal reflexes have returned, usually after 4-6 weeks of injury. Adrenergic receptor up-regulation and abnormal synaptic connections due to postinjury sprouting are the other suggested pathophysiological mechanisms. The clinical manifestations include: an increase in blood pressure of at least 20%, headache, nausea, blurred vision, flushing, sweating, chills, nasal congestion, conjunctival congestion, pallor, and piloerection. Make the patient comfortable, remove any restrictive clothing, and look for and rule out bladder distension and constipation. Blocked urinary catheters or impacted feces must be addressed. Every effort should be made to search for the cause, and the priority of management should be the removal of the precipitating stimulus for ADR.
Follow up rehabilitation
After addressing the systemic injuries and weaning off spinal shock, all SCI patients need rehabilitation in a neuro-intensive care unit53). The purpose of SCI rehabilitation is to treat all compromised systems in order to help the patient regain as much independence as possible. In order to prevent pneumonia and atelectasis, the pulmonary system needs to be carefully monitored with frequent mobilization and deep inspirometry. Patients who are unable to turn themselves in bed require regular turning every two hours and the use of air mattresses to reduce pressure on these areas. Teaching patients to perform frequent self-catheterizations is part of genitourinary treatment. Suppositories and stool softeners should be used with bowel training. For the patient to adjust psychologically, support and counseling are necessary. A long-term stay of 3-6 months is usually required at specialized SCI rehabilitation facilities to achieve these objectives.
CONCLUSION
Anaesthetic management for various systemic injuries requiring surgery in SCI patients with spinal shock is challenging. A thorough understanding of the patients' cardiovascular and respiratory physiology is critical for preventing or limiting secondary SCI and improving postoperative outcomes. A skilled anesthesiologist with a proper plan for airway management while maintaining spinal alignment is crucial. A multidisciplinary team approach, along with good communication among the operative team and neuromonitoring team members, is warranted for the successful management of these patients.
Notes
Ethics statement
This study was a literature review of previously published studies and was therefore exempt from institutional review board approval.
Author contributions
Conceptualization, Formal analysis: Sri Rama Ananta Nagabhushanam Padala, Vaishali Waindeskar, Jai Prakash Sharma, Amit Agrawal, Seema. Data curation: All authors. Writing – original draft: Sri Rama Ananta Nagabhushanam Padala, Molli Kiran, Vaishali Waindeskar, Amit Agrawal, Seema. Writing – review & editing: Molli Kiran, Jai Prakash Sharma, Amit Agrawal, Seema.
Conflict of interest
There is no conflict of interest to disclose.
Funding
None.
Data availability
None.
Acknowledgements
None.