Individualized Periprocedural and Intraprocedural Management for Coil Embolization of Unruptured Cerebral Aneurysm in a Patient with Severe Thrombocytopenia due to Liver Cirrhosis
Article information
Abstract
A female patient in her early 60's with advanced liver cirrhosis and thrombocytopenia having a platelet count under 30,000/µL, visited an outpatient clinic. Computed tomography angiography revealed an unruptured aneurysm on the left middle cerebral artery bifurcation with 6.0 mm of maximal diameter. Endovascular coiling was planned, considering the patient's medical condition. Eight pints of platelet concentrate were transfused 6 hours before the procedure, and a platelet count of 57,000/ µL was achieved 3 hours before the procedure. No antiplatelet premedication and intraprocedural heparin was administered. A bail-out use of intra- or postoperative Tirofiban (Aggrastat®) was prepared for inadvertent thromboembolic events. The aneurysm was successfully occluded with no procedure-related event. The patient’s hospital course was uneventful, and she was discharged without complications. Despite the potential hemorrhagic risk caused by hemostatic failure in patients with cirrhosis, successful endovascular coiling can be performed with highly individualized management.
INTRODUCTION
Preprocedural antiplatelet therapy and intraprocedural anticoagulation are widely accepted methods to avoid thromboembolic events that may occur during or after an endovascular procedure for unruptured intracranial aneurysms1). However, in a particular population lacking adequate hemostasis, there may be a hesitation in adopting the conventional periprocedural antithrombotic management concerning the procedure-related hemorrhagic risk. Thrombocytopenia and hemostatic failure are common conditions in liver cirrhosis (LC) patients2). Although many studies have assessed procedure-related bleeding risk and periprocedural management in LC patients, there are no established guidelines for periprocedural hemostatic management in LC patients with thrombocytopenia, especially before neurointerventional procedures2). Herein, we report a case of successful endovascular coiling of an unruptured intracranial aneurysm (UIA) in a LC patient with severe thrombocytopenia. We also studied the appropriate periprocedural and intraprocedural management for UIA coiling in LC patients with hemostatic defects.
CASE
A female patient in her early 60s visited the outpatient department complaining of a chronic headache. Computed tomography (CT) angiography revealed a saccular aneurysm at the bifurcation of the left middle cerebral artery with 6.0 mm of maximal diameter and 3.8mm of neck size (Fig. 1). The patient had nonalcoholic decompensated LC with a Child–Turcotte–Pugh score of 8 and was, categorized as class B. Laboratory tests revealed severe thrombocytopenia (30,000cells/µL), International normalized ratio (INR) of 1.22, activated partial thromboplastin time (aPTT) of 38.3 seconds (normal range of 28.0–45.0 seconds in our institution), and a fibrinogen level of 230 mg/dL (normal range of 190–450 in our institution). Considering the life expectancy of this patient with decompensated LC, observation of this unruptured aneurysm was also an option. However, the decision to treat the aneurysm was made due to the expectations of liver transplantation and the patient's fear of aneurysmal rupture. Endovascular coiling was planned, considering the patient's medical condition.
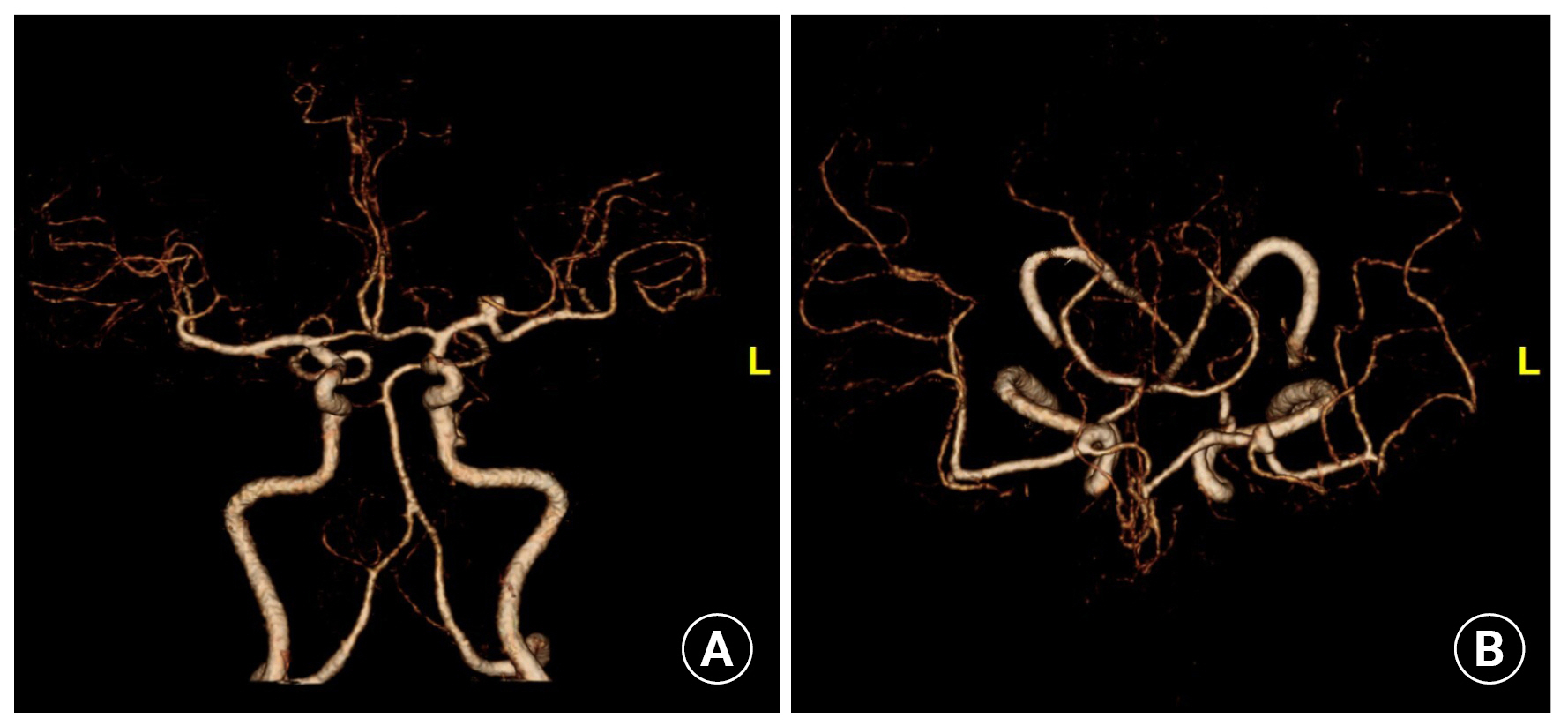
(A) Anterior-posterior view and (B) dorsal view of computed tomography angiography revealed a saccular aneurysm at the bifurcation of the left middle cerebral artery.
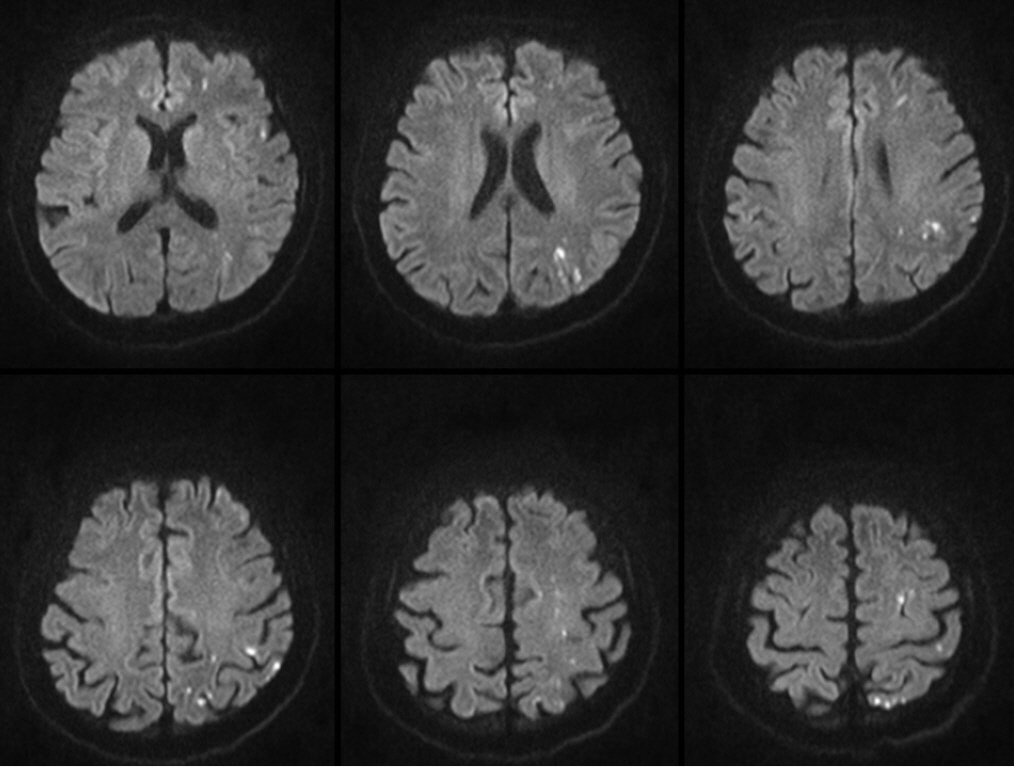
The traditional thresholds of platelet count > 50,000/µL and INR < 1.5 have been accepted for the safety of major surgery3). Based on literature on successful endovascular coiling of UIA in a patient with idiopathic thrombocytopenic purpura (ITP) with periprocedural platelet count of 53,000/µL, we decided to normalize the thrombocytopenia as much as possible, aiming for a platelet count > 50,000/µL4). Detailed blood transfusion procedures were performed in accordance with the Korean domestic blood transfusion guidelines. Eight pints of platelet concentrate were transfused 6 hours before the procedure, and a platelet count of 57,000/µL was achieved 3 hours before the procedure. No perioperative antiplatelet therapy was administered. Considering the shape of the aneurysm based on the CT angiography findings, simple coiling without an adjunctive device was planned. Off-label, bail-out use of Tirofiban (Aggrastat®) administration was prepared if rescue stenting was required or an inadvertent intraprocedural thromboembolic event occurred. Coiling was performed under general anesthesia, and systemic heparinization was not performed. Heparinized saline was used only for device preparation. A 6 French Envoy DA XB (Cordis, Miami Lakes, FL, USA) guiding catheter was advanced through a 7 French femoral sheath (Terumo, Tokyo, Japan). The aneurysm was selected using an SL-10 pre-shaped S microcatheter (Stryker, Fremont, CA, USA). Catheters and wires were navigated with extreme caution. Six detachable coils (Stryker, Fremont, CA, USA) were deployed, and complete aneurysm occlusion was achieved (Fig. 2). The arterial puncture site was closed using a suture-mediated device (Perclose; Abbott Vascular, CA, USA). The procedure was performed successfully without any copmlications. Diffusion magnetic resonance (MR) image acquired on the first postoperative day revealed multiple high embolic signals; however, the patient was asymptomatic (Fig. 3). The hospital course was uneventful, and the patient was discharged with a modified Rankin Scale score of 0. A 6-month follow-up MR angiography showed no evidence of recanalization, and the patient's general medical condition remained unchanged.
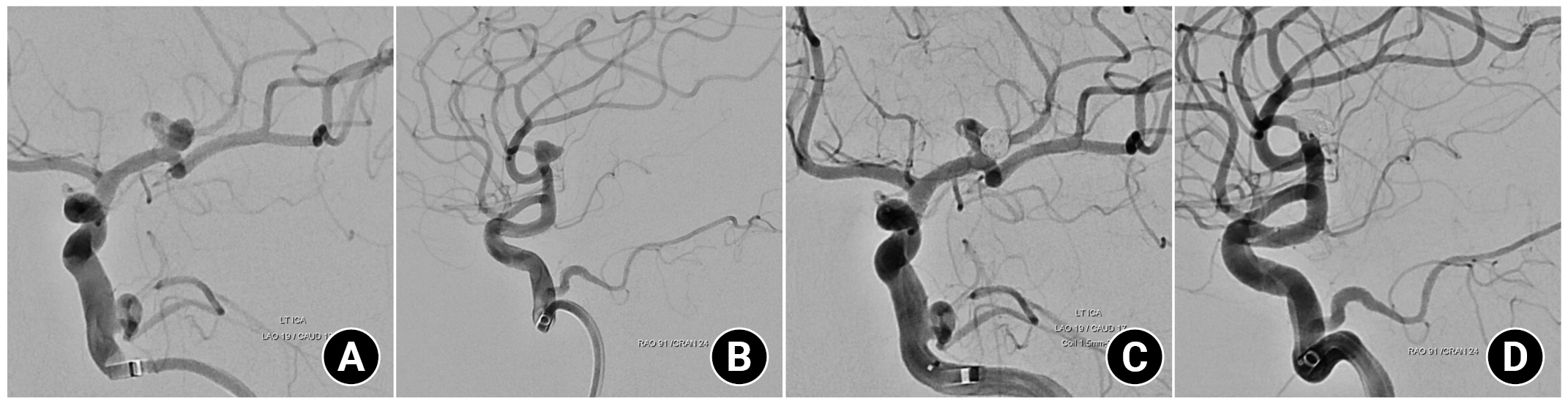
(A) Working angle angiogram of anterior-posterior view and (B) Lateral view before coil packing, (C) Final angiogram of anterior-posterior view and (D) Lateral view showed complete occlusion of aneurysm.
DISCUSSION
Periprocedural management of thrombocytopenia in neurointervention
Literature on elective coil embolization of UIA in patients with thrombocytopenia is scarce4). Ishihara et al. reported endovascular trapping of a vertebral artery fusiform aneurysm in a patient with ITP4). That patient received glucocorticoid and high-dose gamma globulin to correct the platelet count from 29,000/µL to 53,000/µL, and the aneurysm was successfully occluded without complication4).
Even in the whole field of neuroendovascular procedures, few studies have been conducted in patients with thrombocytopenia. Several studies on patients with acute ischemic stroke (AIS) and thrombocytopenia who underwent mechanical thrombectomy (MT), have reported that patients with thrombocytopenia did not suffer an increased risk of symptomatic intracerebral hemorrhage compared with those with normal platelet counts5-7). However, patients with severe thrombocytopenia, with platelet count < 50,000/µL, were rarely identified or unspecified in similar studies5-7). The expert opinion from the recently published Society of Neurointerventional Surgery guideline on MT for AIS patients is that hemorrhagic complication in patients with platelet count < 20,000/µL is concerning8). The guidelines also state that platelet transfusion for patients with a very low platelet count may be considerable8). However, a clear cut-off level for platelet count is not provided in the guideline8).
Preprocedural hemostatic management for LC-induced coagulopathy
The consensus of the hemostatic goal for the high-risk procedure, including the arterial intervention of the central nervous system (CNS), is the same as the traditional thresholds of platelet count > 50,000/µL and INR < 1.53). Consideration of platelet transfusion and correction of INR were also suggested but were only weakly recommended3). In LC patients who undego pathological changes in both the procoagulant and anticoagulant pathways, the hemostatic condition cannot be accurately assessed by traditional laboratory tests, such as INR, aPTT, and platelet count2,3). Therefore, preprocedural hemostasis guidelines recommend that physicians make judicious decisions of prophylactic preprocedural platelet transfusion in patients with cirrhosis2,3). This recommendation was extracted from studies on low-risk procedures, such as thoracentesis and paracentesis, the absence of CNS procedures, or unknown whether CNS or not9-11).
Therefore, whether these results opposing platelet transfusion can be wholly adapted to neurointervention is questionable. Regardless of its volume and location, a hemorrhage within the CNS can be fatal. Reflecting this unique situation of the CNS, individualized decision-making regarding platelet transfusion for LC patients before high-risk procedures is recommended2,3).
Prophylactic fresh frozen plasma for LC patients even before a high-risk procedure is not recommended because the risk of circulatory overload outweighs the benefit of its minimal increment in hemostasis2,3). The administration of thrombopoietin receptor agonists and 1-Deamino-8-d-arginine vasopressin to prevent procedure-related bleeding is discouraged due to insufficient evidence from studies on small sample size2). Only cryoprecipitate can be used to correct plasma fibrinogen levels > 100 mg/dL before a high-risk procedure2).
Antithrombotic use for UIA coiling in patients with hemostatic failure
Before high-risk procedures for patients with cirrhosis, antithrombotics should be discontinued without bridging therapy unless there is an evident thrombotic risk, such as a high risk of venous thromboembolism or a presence of a mechanical heart valve, not exclusively for LC3). In current practice, antiplatelet therapy is typically administered when stenting is planned and is not always used before coiling without an adjunctive device12). Therefore, we omitted preprocedural antiplatelet agents for LC patients with severe thrombocytopenia in this study.
Generally, systemic heparinization is a preventive measure for thromboembolic events during procedures for UIAs1). However, heparin use for patients with cirrhosis has extremely limited indication and is contraindicated in patients with platelet count < 100,000/µL13). Even in situation of severe LC complication such as portal vein thrombosis, unfractionated heparin has been rarely used2). In our study, unfractionated heparin was used only for device preparation.
Off-label tirofiban use in patients with hemostatic defects
Even in cases of hemostatic failure, thromboembolic event caused by coil mesh or device may be a concern. Tirofiban has been widely used to manage acute thromboembolisms during coil embolization of cerebral aneurysms14). Tirofiban is a short-acting, potent Glycoprotein IIb/IIa receptor inhibitor capable of dissolving even a fresh thrombus15). In studies on continuous intravenous (IV) tirofiban infusion in a cohort of 86 patients during or after neuroendovascular procedures, no significant differences in intracranial hemorrhage were found between in-label and off-label use15). Its safety profile is acceptable even for off-label use beyond the contraindications, including thrombocytopenia (<100,000/μL), aPTT > 1.3-fold, INR > 1.5, and severe liver insufficiency (Child–Pugh class C)15). Considering that the previous report was on IV maintenance infusion, intra-arterial tirofiban injection with a relatively lower dose and a short-acting mechanism seems acceptable for intraprocedural thrombolysis, even in patients with inadequate hemostasis. This study has limitations due to the nature of a case report. However, despite our best efforts of search, published literature on elective UIA coiling in patients with advanced LC and severe thrombocytopenia was scarce. Our study will help patients in similar situations of coagulopathy. And we raised the need for further research on intra- and periprocedural antithrombotic strategies in patients with hemostatic failure who require neurointerventional procedures.
CONCLUSIONS
A highly individualized approach is needed for hemostatic management of LC patients with severe thrombocytopenia before UIA coiling. We suggest prophylactic transfusion to achieve a platelet count > 50,000/μL and an INR < 1.5. Bail-out use of tirofiban for inadvertent intra- or postoperative thromboembolism may be considered for patients with hemostatic failure.
Notes
Ethics statement
The study was approved by the Institutional Review Board (IRB) of Sanggye Paik Hospital Hospital (No. SGPAIK 2023-08-015). The retrospective nature of this case report waived the informed consent requirement.
Author contributions
Conceptualization: SYC. Data curation, Methodology, Writing – original draft: HJK, SYC. Formal analysis: SYC. Project administration: SYC. Visualization: SYC. Writing – review & editing: SYC.
Conflict of interest
There is no conflict of interest to disclose.
Funding
None.
Data availability
None.
Acknowledgements
None.