 |
 |
- Search
| J Neurointensive Care > Volume 6(1); 2023 > Article |
|
Abstract
Background
Hemorrhagic Moyamoya disease (MMD) is a common subtype of MMD in adult patients, especially in East Asian countries. To our knowledge, current studies regarding factors affecting the prognosis in patients with hemorrhagic MMD is lacking. This study aimed to determine the potential prognostic factors for hemorrhagic MMD.
Methods
This retrospective study reviewed patients with hemorrhagic MMD diagnosed at our hospital between June 2011 and June 2021. Patient outcome was measured at discharge using the extended Glasgow outcome scale (GOSE). Prognostic factors were assessed by multivariate logistic regression analysis.
Results
Patients discharged with worst outcomes (GOSE 1 and 2) had significantly lower initial Glasgow coma scale (GCS) scores (5.9 ± 3.1 vs. 10.3 ± 3.7; p = 0.002) and were associated with more severe intraventricular hemorrhage (IVH) (p = 0.007), and IVH-related hydrocephalus requiring extraventricular drain (EVD) insertion was more common (n = 12 vs. n = 7; p = 0.001). The multivariate analysis showed that IVH-related hydrocephalus requiring EVD placement (OR 8.256, 95% CI 0.996–68.450; p = 0.016) and low initial GCS scores (OR 0.644; 95% CI 0.465–0.892; p = 0.008) were independent risk factors for unfavorable outcomes (GOSE 1–4) in patients with hemorrhagic MMD.
Moyamoya disease (MMD) is a unique disease entity characterized by gradual occlusion of the bilateral distal internal carotid arteries and secondary collateral vessel network formation of unknown etiology. Hemorrhagic MMD is a common subtype, particularly in adult patients, and has different clinical profiles from ischemic MMD1,2). Specifically, patients with hemorrhagic MMD are more likely to experience serious neurologic damage and consequently show worse prognosis3,4). Surgical revascularization and other medical treatments may be beneficial for patients with MMD; however, these treatment options can only be considered if they show favorable outcomes. Therefore, the prediction of disease prognosis in patients with hemorrhagic MMD is valuable.
The associated hemorrhage can be classified into intraparenchymal hemorrhage (IPH), intraventricular hemorrhage (IVH), and subarachnoid hemorrhage (SAH). MMD-related spontaneous intracerebral hemorrhage (ICH) is different from primary spontaneous ICH in that the hemorrhage volume is usually less and the location frequently involves the ventricle5). A previous study suggests that in patients with hemorrhagic MMD with IVH, IVH severity measured by the Graeb scale is an independent risk factor for poor short-term outcomes3). However, only a few studies have focused on factors associated with hemorrhagic MMD, and, in this study, we analyzed several disease-related and patient-related prognostic factors of this unique disease entity.
This single-center, retrospective study analyzed patients diagnosed with MMD and presented with hemorrhagic episodes between June 2011 and June 2021. Patients were included if 1) IPH, IVH, and SAH were seen on the initial non-contrast computed tomography (CT) and 2) CT angiography (CTA) and/or digital subtraction angiography (DSA) confirmed MMD. Patients were excluded if 1) diagnosed and/or received surgical treatment before our study period at another hospital, 2) associated with ischemic MMD, and 3) had a history of other brain lesions such as brain tumors, traumatic brain injury, and vasculitis.
In this study, MMD was defined as 1) stenosis or occlusion of the terminal portion of the intracranial internal carotid artery or proximal portions of the anterior and/or the middle cerebral artery and 2) not associated with (i) atherosclerosis, (ii) autoimmune disease, (iii) meningitis, (iv) brain tumors, (v) Down syndrome, (vi) head injury, and (vii) cerebrovascular lesions after head irradiation6). Patients with probable MMD with unilateral vessel involvement were not excluded.
Clinical data were obtained by reviewing the electronic medical records. The following data were collected: age, sex, initial Glasgow coma scale (GCS) score, family and medical histories (hypertension, diabetes mellitus [DM], hyperlipidemia, smoking, and alcohol abuse). A patient questionnaire was used upon admission, and self-medication lists were reviewed to confirm these underlying conditions. Only those who never smoked in their lifetimes were called non-smokers. Lifelong abstainers who never drank alcohol in the past and drinkers who drank less than once a month were categorized as the non-alcohol consumption group.
At the time of discharge, the patient outcome was measured with an extended Glasgow outcome scale (GOSE). Worst outcomes were considered for GOSE 1 and 2 and unfavorable outcomes for GOSE 1–4. Operation records, skull X-ray images, and brain CT images were reviewed to confirm surgical procedures, including extraventricular drainage (EVD) catheter placement.
All patients underwent non-contrast brain CT upon admission, and CTA and/or DSA were performed afterward to confirm MMD. Four patients had general conditions which were too critical to perform DSA. All CT images were analyzed for the presence of IPH, IVH, and SAH.
IPH volume was calculated using the ABC/2 formula, and the Graeb scale was used to determine IVH severity (Fig. 1). The location of hemorrhage was also analyzed and categorized as follows: 1) basal ganglia ± IVH, 2) thalamus ± IVH, 3) IVH only, and 4) others (Fig. 2). Recurrent hemorrhage was diagnosed if follow-up CT scans showed volume expansion greater than 5 mL and/or new hemorrhage development at another location. Hydrocephalus was diagnosed on initial CT with the following criteria: 1) Evans index greater than 0.3 and 2) enlargement of the anterior horn, temporal horn, third ventricle of the lateral ventricle, and periventricular interstitial edema.
Continuous variables are expressed as mean ± SD. Categorical variables are expressed as frequencies and percentages. To clarify the differences between two outcome groups, statistical analyses using independent t tests, chi-square tests, and Fisher’s exact tests were conducted. Independent t tests were run on continuous variables, and chi-square tests were used for categorical variables. If more than 20% of the cells in the chi-square test had an expected frequency of less than 5, the likelihood ratio or Fisher’s exact test was used. Univariate and multivariate logistic regression were performed to analyze the association between patient outcomes and potential prognostic factors. Factors with p-value < 0.10 in the univariate analysis were included in the multivariate model. All statistical analyses were performed using IBM SPSS Statistics version 23.0 (IBM Corp., Armonk, NY, USA). Statistical significance was defined as a p-value < 0.05.
Between June 2011 and June 2021, a total of 37 patients with hemorrhagic MMD were admitted to our hospital. Among them, 26 were female (70.3%) and 11 were male (29.7%). The mean age was 56 years, ranging from 13 to 82 years. During their treatment, 11 patients expired (GOSE 1), and 3 patients were confirmed vegetative (GOSE 2) at discharge. Nine patients expired due to increased intracranial pressure (IICP), and two patients due to sepsis. Furthermore, nine patients were discharged with severe functional disability (GOSE 3 and 4).
Patients discharged with worst outcomes (GOSE 1 and 2) had significantly lower initial GCS scores (5.9 ± 3.1 vs. 10.3 ± 3.7; p = 0.002) and were associated with more severe IVH (p = 0.007), and IVH-related hydrocephalus requiring EVD insertion was more common (n = 12 vs. n = 7; p = 0.001). Moreover, underlying DM (n = 6 vs. n = 2; p = 0.014) and hyperlipidemia (n = 4 vs. n = 1; p = 0.037) were also more frequently observed than those who were conscious at discharge (GOSE 3–8). Although not statistically significant, patients with worst outcomes were generally older (60.0 ± 9.5 vs. 53.6 ± 14.9 years; p = 0.158) and presented with larger IPH volume (48.7 ± 48.6 mL vs. 31.47 ± 31.1 mL, p = 0.271). No statistically significant difference was found between the two groups in terms of the female sex, hemorrhage location, hemorrhage recurrence, IVH, underlying hypertension, and smoking/alcohol abuse history (Table 1).
Patients discharged with unfavorable outcomes (GOSE 1–4) also showed significantly lower initial GCS scores (6.5 ± 3.0 vs. 12.1 ± 3.1; p < 0.001) than those with favorable outcomes (GOSE 5–8). In addition, recurrent hemorrhage (n = 6 vs. n = 0; p = 0.037), IVH (n = 22 vs. n = 10; p = 0.037) and IVH-related acute hydrocephalus requiring EVD insertion (n = 17 vs. n = 2; p = 0.000) were more common, and IVH was also more severe (p = 0.037) in this group. Although not statistically significant, patients with unfavorable outcomes were older (57.4 ± 12.8 years vs. 53.7 ± 14.5 years; p = 0.424) and presented with larger IPH volume (47.9 ± 42.8 mL vs. 20.8 ± 22.8 mL, p = 0.078). No statistically significant difference was found between the two groups in terms of the female sex, hemorrhage location, and underlying medical conditions (Table 2).
The multivariate analysis showed that only a low initial GCS score (odds ratio [OR] 0.486; 95% confidence interval [CI] 0.273–0.864; p = 0.014) was associated with worst outcomes (GOSE 1 and 2). IVH-related hydrocephalus requiring EVD placement (OR 8.256, 95% CI 0.996–68.450; p = 0.016) and low initial GCS scores (OR 0.644; 95% CI 0.465–0.892; p = 0.008) were independent risk factors for unfavorable outcomes (GOSE 1–4) in patients with hemorrhagic MMD (Tables 3, 4). To determine the accuracy and best cutoff GCS score, the ROC curve was obtained, and an initial GCS score of <8 (p < 0.001) was sufficiently sensitive and specific to predict poor outcomes (Fig. 3).
In this retrospective study of 37 patients, we analyzed the potential prognostic factors of hemorrhagic MMD. Several significant clinical differences between patients with good outcomes and poor outcomes were noted, and these were GCS scores on admission, IVH, IVH severity measured by the Graeb scale, IVH-related hydrocephalus requiring EVD insertion, recurrent hemorrhage, DM, and hyperlipidemia. Among these factors, the multivariate analysis showed that an initial GCS score of <8 and IVH-related hydrocephalus requiring EVD insertion were two independent risk factors associated with poor outcomes at discharge. These findings provide valuable information regarding the disease prognosis and may guide clinicians in their therapeutic decision-making process for patients with hemorrhagic MMD.
Our result is compatible with those of several previous studies, suggesting that the degree of neurologic impairment based on the initial GCS score is a strong predictor of ICH outcome7,8). Furthermore, IVH accompanied by ICH is a known poor prognostic factor with expected mortality between 50% and 80%9). In this study, IVH severity, as calculated based on the Graeb score, differed significantly between patients with favorable and unfavorable outcomes at discharge. This may be due to the fact that IICP induced by severe IVH may result in inflammation and secondary brain injury that could further aggravate vicious IICP cycle. In a recent study, Tong et al. analyzed MMD with IVH in adults and reported that a higher Graeb score was a significant risk factor for poor prognosis (mRS > 2); however, our multivariate analysis showed no statistically significant relationship between these variables3). Moreover, in their study on the short-term prognostic factors for adult patients with hemorrhagic MMD, Yu et al. suggested SAH and IPH as predictors of poor functional outcomes; however, we found no statistically significant associations10). Such disparity may be due to our small sample size; thus, further prospective study with a larger cohort is needed to confirm our study findings.
IVH can disrupt cerebrospinal fluid absorption, causing acute obstructive hydrocephalus, increased intracranial pressure, and neurological deterioration11). In some studies, early surgical interventions such as EVD insertion or craniotomy and hematoma removal have shown protective effects and improved functional outcomes at short-term compared with conservative treatment3,12). However, our data suggest that IVH-related acute hydrocephalus requiring EVD placement is an independent risk factor related to poor prognosis at discharge. Such difference may be due to the fact that our analysis of functional outcomes was based on the time of discharge, and further extended follow-up period could suggest different outcomes.
Among the 37 patients with hemorrhagic MMD, recurrent bleeding was seen in 6 (16.2%), and patients with GOSE 1–4 (unfavorable prognosis) at discharge were more frequently associated with rebleeding than those with GOSE 5–8 (favorable prognosis). This result is consistent with previous study suggesting that clinical outcomes are expected to be worse after recurrent bleeding13). Although radiologic characteristics and underlying hemodynamic status were not considered in our study because of insufficient data, previous studies have suggested that reduced basal perfusion, and Suzuki stage progression may play a pivotal role in ICH recurrence in patients with MMD13). Therefore, thorough hemodynamic evaluation is required, especially for patients in favorable conditions, as they may be candidates for revascularization.
Previous studies have reported that age is associated with the incidence of hemorrhagic MMD6,14,15), and our result, although statistically insignificant, suggests that older patients may suffer worse outcomes than their younger counterparts. In addition, hyperlipidemia has been recognized in patients with Moyamoya disease, with an incidence of 27.7% in a study performed in the Mayo Clinic Minnesota 1979–2011 and 37.3% in a study done in Japan 2001–20111). Although the relationship between cholesterol level and the risk of hemorrhagic stroke is inconclusive, several previous studies have shown that total cholesterol level was inversely associated with the risk of hemorrhagic stroke, as low cholesterol levels may contribute to the development of a fragile endothelium, prone to leakage and rupture16-19). In this study, underlying hyperlipidemia appeared to be related to poor clinical outcomes, and we hypothesize that this result is partly because Moyamoya vessels are more prone to atherosclerosis caused by high serum cholesterol levels, which may make patients be more susceptible to secondary insult after hemorrhage. Further prospective studies with a larger study population are needed to clearly understand the complex pathophysiology underlying cholesterol level and cerebrovascular diseases.
This study has some limitations. First, the sample size is small, partly because the participants were selected from a single institute. Second, this is a retrospective study. Third, patients’ clinical outcomes were measured at discharge, and long-term follow-up data were not included. Fourth, factors including medical conditions and radiological and hemodynamic data such as Suzuki grade and brain perfusion were not evaluated because of inadequate data. All these limitations could affect the accuracy of our results.
In this study, we analyzed several potential prognostic factors for patients with hemorrhagic MMD. An initial GCS score of <8 and IVH-related hydrocephalus requiring EVD insertion were independent risk factors associated with poor outcomes at discharge. In clinical practice, these factors should be considered in the therapeutic decision-making process. Further prospective studies with a larger study population and more randomized designs are warranted to confirm our findings.
Fig. 2.
Hemorrhage location. (A) Basal ganglia ± IVH*, (B) thalamus ± IVH, (C) IVH only, and (D) others * IVH: intraventricular hemorrhage.
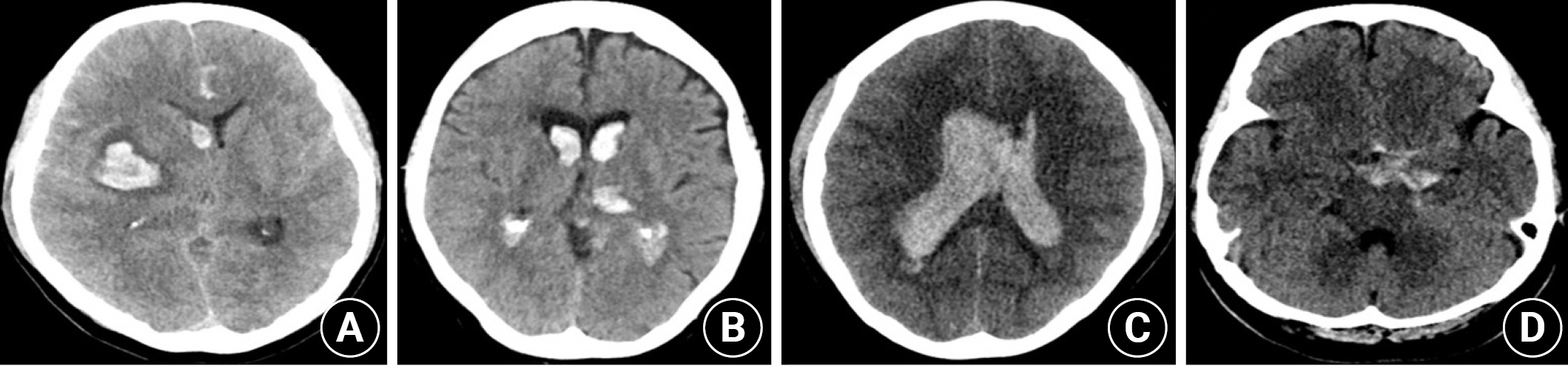
Fig. 3.
Receiver operating characteristic (ROC) curve of the Glasgow coma scale (GCS) score for (A) Worst outcome (GOSE 1–2), (B) Unfavorable outcome (GOSE 1–4). The area under the curve reflects the performance of the GCS to predict unfavorable outcomes.

Table 1.
Comparison between patients with extended Glasgow outcome scale (GOSE) 1–2 and GOSE 3–8 at discharge
| GOSE 1,2 (n=14) | GOSE 3-8 (n=23) | p-value | |
|---|---|---|---|
| Age | 60.0 ± 9.5 | 53.6 ± 14.9 | 0.158 |
| Female (n=26) | 10 | 16 | 0.904 |
| Initial GCS | 5.9 ± 3.1 | 10.3 ± 3.7 | 0.002* |
| GCS 13-15 (n=9) | 1 | 8 | |
| GCS 9-12 (n=7) | 0 | 7 | |
| GCS 3-8 (n=21) | 13 | 8 | |
| Hemorrhage location | 0.841 | ||
| Basal ganglia ± IVH (n=17) | 7 | 10 | |
| Thalamus ± IVH (n=3) | 1 | 2 | |
| IVH only (n=9) | 4 | 5 | |
| Others (n=8) | 2 | 6 | |
| IPH (n=27) | 48.7 ± 48.6 | 31.47 ± 31.1 | 0.271 |
| 0 < Volume < 20mL (n=11) | 3 | 8 | 0.384 |
| 20mL < Volume < 40mL (n=5) | 2 | 3 | 0.879 |
| 40mL < Volume < 60mL (n=4) | 2 | 2 | 0.561 |
| 60mL < Volume (n=7) | 3 | 4 | 0.711 |
| Recurrent hemorrhage (n=6) | 4 | 2 | 0.112 |
| IVH (n=32) | 14 | 18 | 0.061 |
| Severe IVH (Graeb score > 8, n=16) | 10 | 6 | 0.007* |
| EVD insertion (n=19) | 12 | 7 | 0.001* |
| Aneurysm (n=3) | 1 | 2 | 0.867 |
| Hypertension (n=17) | 7 | 10 | 0.699 |
| Diabetes Mellitus (n=8) | 6 | 2 | 0.014* |
| Hyperlipidemia (n=5) | 4 | 1 | 0.037* |
| Smoking (n=5) | 2 | 3 | 0.915 |
| Alcohol (n=8) | 1 | 7 | 0.095 |
Table 2.
Comparison between patients with extended Glasgow outcome scale (GOSE) 1–4 and GOSE 5–8 at discharge
| GOSE 1-4 (n=23) | GOSE 5-8 (n=14) | p-value | |
|---|---|---|---|
| Age | 57.4 ± 12.8 | 53.7 ± 14.5 | 0.424 |
| Female (n=26) | 16 | 10 | 0.904 |
| Initial GCS | 6.5 ± 3.0 | 12.1 ± 3.1 | < 0.001* |
| GCS 13-15 (n=9) | 1 | 8 | |
| GCS 9-12 (n=7) | 4 | 3 | |
| GCS 3-8 (n=21) | 18 | 3 | |
| Hemorrhage location | 0.443 | ||
| Basal ganglia ± IVH (n=17) | 12 | 5 | |
| Thalamus ± IVH (n=3) | 2 | 1 | |
| IVH only (n=9) | 6 | 3 | |
| Others (n=8) | 3 | 5 | |
| IPH (n=27) | 47.9 ± 42.8 | 20.8 ± 22.8 | 0.078 |
| 0mL < Volume < 20mL (n=11) | 5 | 6 | 0.118 |
| 20mL < Volume < 40mL (n=5) | 3 | 2 | 0.879 |
| 40mL < Volume < 60mL (n=4) | 3 | 1 | 0.589 |
| 60mL < Volume (n=7) | 6 | 1 | 0.148 |
| Recurrent hemorrhage (n=6) | 6 | 0 | 0.037* |
| IVH (n=32) | 22 | 10 | 0.037* |
| Severe IVH (Graeb score > 8, n=16) | 13 | 3 | 0.037* |
| EVD insertion (n=19) | 17 | 2 | <0.001* |
| Aneurysm (n=3) | 1 | 2 | 0.283 |
| Hypertension (n=17) | 11 | 6 | 0.769 |
| Diabetes Mellitus (n=8) | 7 | 1 | 0.095 |
| Hyperlipidemia (n=5) | 4 | 1 | 0.377 |
| Smoking (n=5) | 3 | 2 | 0.915 |
| Alcohol (n=8) | 4 | 4 | 0.423 |
Table 3.
Multivariate analysis for extended Glasgow outcome scale (GOSE) 1–2
| OR | 95% C.I | p-value | |
|---|---|---|---|
| GCS | 0.486 | 0.273–0.864 | 0.014* |
| IVH (Graeb score > 8) | 0.729 | 0.367–1.449 | 0.368 |
| EVD insertion | 7.879 | 0.703–88.333 | 0.094 |
| DM | 17.088 | 0.393–742.840 | 0.140 |
| Hyperlipidemia | 123.485 | 0.769–19835.608 | 0.063 |
REFERENCES
1. Fujimura M, Tominaga T. Hemorrhagic moyamoya disease: a recent update. J Korean Neurosurg Soc 2019;62:136–143.




3. Tong P, Shan T, An J, Liu S, Jing G, Bi J, et al. Analysis of clinical characteristic and risk factors for short-term prognosis of moyamoya disease with intraventricular hemorrhage in adults. World Neurosurg 2023;171:e738–e744.


4. Yang H, Zhang L, Wang M, Wang J, Chen L, Lu H. Clinical features of and risk factors for intracranial aneurysms associated with moyamoya disease. Int J Stroke 2021;16:542–550.



5. Nah HW, Kwon SU, Kang DW, Ahn JS, Kwun BD, Kim JS. Moyamoya disease-related versus primary intracerebral hemorrhage: [corrected] location and outcomes are different. Stroke 2012;43:1947–1950.


6. Research committee on the pathology and treatment of spontaneous occlusion of the circle of Willis: guidelines for diagnosis and treatment of Moyamoya disease (spontaneous occlusion of the circle of Willis). Neurol Med Chir 2012;52:245–266.
7. Maas MB, Francis BA, Sangha RS, Lizza BD, Liotta EM, Naidech AM. Refining prognosis for intracerebral hemorrhage by early reassessment. Cerebrovasc Dis 2017;43:110–116.



8. Safatli DA, Gunther A, Schlattmann P, Schwarz F, Kalff R, Ewald C. Predictors of 30-day mortality in patients with spontaneous primary intracerebral hemorrhage. Surg Neurol Int 2016;7:S510–517.



9. Hinson HE, Hanley DF, Ziai WC. Management of intraventricular hemorrhage. Curr Neurol Neurosci Rep 2010;10:73–82.




10. Yu Z, Zheng J, Liu X, Wen D, Guo R, Li M, et al. Prognostic factors for adult patients with hemorrhagic moyamoya disease in the acute stage. Clin Neurol Neurosurg 2019;184:105409.


11. Montiel V, Grandin C, Goffette P, Fomekong E, Hantson P. Refractory high intracranial pressure following intraventricular hemorrhage due to Moyamoya disease in a pregnant caucasian woman. Case Rep Neurol 2009;1:1–7.



12. Mendelow AD, Gregson BA, Rowan EN, Murray GD, Gholkar A, Mitchell PM, et al. Early surgery versus initial conservative treatment in patients with spontaneous supratentorial lobar intracerebral haematomas (STICH II): a randomised trial. Lancet 2013;382:397–408.



13. Jo KI, Kim MS, Yeon JY, Kim JS, Hong SC. Recurrent bleeding in hemorrhagic Moyamoya disease: Prognostic implications of the perfusion status. J Korean Neurosurg Soc 2016;59:117–121.



14. Sato Y, Kazumata K, Nakatani E, Houkin K, Kanatani Y. Characteristics of Moyamoya disease based on National Registry Data in Japan. Stroke 2019;50:1973–1980.


15. Zhang Q, Zhao M, Ge P, Liu X, Wang R, Zhang Y, et al. Hemorrhagic patterns and their risk factors in patients with moyamoya disease. Eur J Neurol 2020;27:2499–2507.



16. Chen YW, Li CH, Yang CD, Liu CH, Chen CH, Sheu JJ, et al. Low cholesterol level associated with severity and outcome of spontaneous intracerebral hemorrhage: results from Taiwan Stroke Registry. PLoS One 2017;12:e0171379.



17. Wang X, Dong Y, Qi X, Huang C, Hou L. Cholesterol levels and risk of hemorrhagic stroke: A systematic review and meta-analysis. Stroke 2013;44:1833–1839.


- TOOLS
-
METRICS

-
- 0 Crossref
- 1,644 View
- 21 Download
- Related articles in JNIC
-
Analysis of Nitrogen Balance Test in Patients With Traumatic Brain Injury2023 April;6(1)






