Acute Kidney Injury Following Mannitol Infusion in Neurosurgical Patients
Article information
Abstract
Background
To evaluate predictors of acute kidney injury (AKI) and their predictive performance during mannitol infusion, and the impact of AKI on in-hospital mortality of neurocritically ill patients.
Methods
This was a retrospective, observational study of patients who were admitted at a tertiary university hospital, Seoul, Republic of Korea, neurosurgical intensive care unit (ICU) from January 2013 to December 2019. We included neurosurgical patients on mannitol infusion admitted in the ICU The primary endpoint was the occurrence of AKI.
Results
A total of 3,964 patients were included in the final analysis. AKI was detected in 540 (13.6%) patients on mannitol infusion. Measured osmolality and osmolar gap were significantly higher in patients with AKI than those without (both p< 0.001). However, the predictive power of the two indicators was similar and were both weak predictors of AKI (both C-statistic <0.650). In the multivariable analysis, maximal measured osmolality, chronic kidney disease, Acute Physiology and Chronic Health Evaluation II score on ICU admission, use of vasopressor, use of glycerin, mechanical ventilation, and invasive ICP monitoring were significantly associated with AKI. In-hospital mortality was significantly higher in patients with AKI than those without (11.1% vs. 1.4%, p< 0.001).
Conclusion
Based on our findings, kidney injury may be associated with poor clinical outcomes in neurosurgical and neurocritically ill patients, and monitoring serum osmolality and osmolar gap remains important in the prevention of kidney injury for patients on mannitol infusion. Moreover, clinical factors related to ICU management and pre-existing renal disease may aggravate AKI during mannitol infusion.
INTRODUCTION
Mannitol infusion is commonly used in the treatment of cerebral edema and elevated intracranial pressure (ICP) in patients with traumatic brain injury, ischemic stroke, intracranial hemorrhage, infection of the central nervous system, and brain tumor1-4). Rapid infusion of high doses of mannitol can effectively reduce ICP5,6) in a dose-dependent manner5,6). However, various complications such as acute kidney injury (AKI), electrolyte imbalance, volume depletion, hypotension, and rebound elevated ICP may occur in long-term use and high doses of mannitol infusion2). While mannitol may show renal protective benefits in specific indications, it has also been implicated in renal toxicity therapy7). The renal toxicity may be aggravated by dehydration or preexisting renal impairment7) and extreme hyperosmolality may cause acute kidney injury (AKI)1). Therefore, it is necessary to monitor electrolytes, blood urea nitrogen, creatine, serum osmolality, and osmolar gap (OG) while using mannitol to prevent AKI and other mannitol infusion side effects2).
However, there are limited well-designed clinical studies that prove the exact relationship between mannitol infusion and kidney injury, and the AKI risk factors during mannitol infusion in neurocritically ill patients1). In this study, we aimed to evaluate the clinical factors related to AKI during mannitol infusion and the impact of AKI on in-hospital mortality of neurocritically ill patients. In addition, we evaluated the effectiveness of serum osmolality and OG in predicting AKI during mannitol infusion.
MATERIALS AND METHODS
Study population and design
This was a retrospective, single-center, observational study of patients who were admitted at the Samsung Medical Center, Seoul, Republic of Korea, neurosurgical intensive care unit (ICU) from January 2013 to December 2019. This study was approved by the Samsung Medical Center Institutional Review Board (IRB) (No. SMC 2020-09-082). Patients’ records were reviewed and analyzed according to the Declaration of Helsinki. The requirement of informed consent was waived by the IRB due to the study’s retrospective nature. We included neurosurgical patients who were on mannitol infusion during their ICU admission and on postoperative management, following brain tumor, subarachnoid hemorrhage, cerebral vascular surgery, intracerebral hemorrhage, cerebral infarction, traumatic brain injury or infection of the central nervous system. The patients’ laboratory data including serum osmolality, sodium, urea, glucose, creatinine, and glomerular filtration rate (GFR) should have been obtained within 7 days from ICU admission. We excluded patients who had end-stage renal disease with renal replacement therapy, insufficient medical records, a ‘do not resuscitation’ order, transferred to other hospitals, or with unknown prognosis.
Definitions and outcomes
In this study, the baseline characteristics such as comorbidities, behavioral risk factors, ICU management, and laboratory data were collected retrospectively using Clinical Data Warehouse. Our center constructed a “Clinical Data Warehouse Darwin-C” designed for the investigators to search and retrieve de-identified medical records from electronic archives.
The laboratory data were obtained at least once daily from all the neurosurgical patients. Assessment of kidney function was based on the GFR using the Modification of Diet in Renal Disease 4-variable formula8). The Acute Kidney Injury Network (AKIN) criteria were used to define perioperative acute renal dysfunction9). “Stage 1” was defined as an increase of creatinine by ≥ 0.3 mg/dL or 1.5–1.9 times, “stage 2” as an increase of creatinine 2.0–2.9 times, and “stage 3” as an increase of creatinine 3 times from baseline (or serum creatinine of more than or equal to 4.0 mg/dL with an acute increase of at least 0.5 mg/dL). Given wide variation in indications and timing of initiation of renal replacement therapy, individuals who receive renal replacement therapy are considered to have met the criteria for stage 3 irrespective of the stage they are in at the time of renal replacement therapy9).Calculated osmolality (mOsm/kg) was defined as: serum sodium level (mEq/L) × 2 + (glucose[mg/dL]/18) + (blood urea nitrogen [mg/dL]/2.8)2). The osmolar gap (OG) was defined as the difference between the measured osmolarity and the calculated osmolality2). End-stage renal disease was defined as on dialysis or GFR < 30 mL/min/1.73 m2for at least 12 weeks10).
In this study, the primary endpoint was the occurrence of AKI9).
Statistical analyses
All data are presented as mean ± standard deviation for continuous variables or frequencies and proportions for categorical variables. Data were compared using Student’s t-test for continuous variables and Chi-square test or Fisher’s exact test for categorical variables. A stepwise multiple logistic regression was performed to obtain a statistically meaningful predictor of in-hospital mortality. Predictive performance of serum osmolality and OG was assessed using areas under the curve (AUCs) of receiver operating characteristic (ROC) curves for sensitivity vs. 1-specificity. AUCs were compared using the nonparametric approach published by DeLong et al.11) for two correlated AUCs. All the tests were two-sided and p values of less than 0.05 were considered statistically significant. All the statistical analyses were performed with R Statistical Software version 4.0.2 (R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
Baseline characteristics
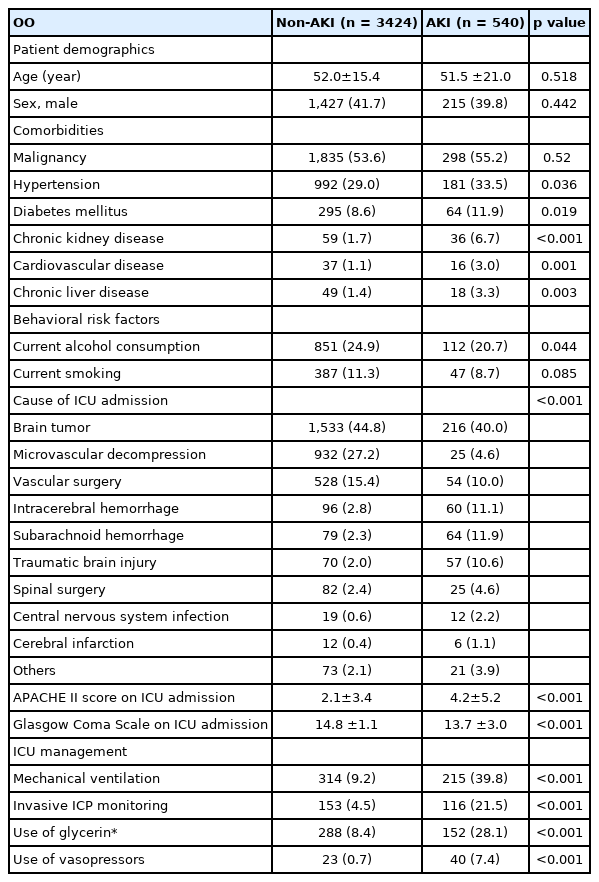
A total of 3,964 patients were included in the final analysis. AKI was detected in 540 (13.6%) patients on mannitol infusion. The mean age of all the patients was 51.9 ± 16.3 years and 41.4% of patients were male. Malignancy (53.8%) and hypertension (29.6%) were the most common comorbidities, while brain tumor (44.1%) was the most common reason for ICU admission (Table 1).
Clinical outcomes of the overall study population
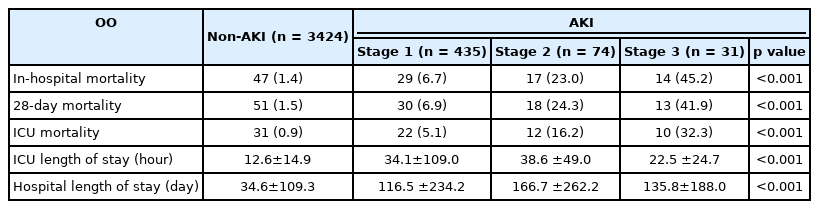
Measured osmolalities were significantly higher in patients with AKI than those without AKI (all p< 0.001).Maximal osmolar gap and creatinine were also significantly higher in patients with AKI than those without (both p< 0.001). GFR was significantly lower in patients with AKI than those without (86.3 ± 35.3 vs.100.0 ± 22.8, p< 0.001) (Table 2). In-hospital and ICU mortality were significantly higher in patients with AKI than those without (11.1% vs. 1.4%, p< 0.001 and 8.1% vs. 0.9%, p< 0.001, respectively). The length of ICU stay was also longer in the AKI group than non-AKI group (124.5±236.1hr vs. 34.6 ±109.3hr, p<0.001) (Table 3). In the multivariable analysis, maximal measured osmolality (adjusted odds ratio [OR]: 1.02, 95% confidence interval [CI]: 1.01 – 1.03), chronic kidney disease (adjusted OR: 2.26, 95% CI: 1.35 – 3.69), Acute Physiology and Chronic Health Evaluation (APACHE) II score on ICU admission (adjusted OR: 1.05, 95% CI: 1.02 – 1.07), use of vasopressor (adjusted OR: 2.86, 95% CI: 1.55 – 5.35), use of glycerin (adjusted OR: 1.72, 95% CI: 1.30 – 2.25), mechanical ventilation (adjusted OR: 2.96, 95% CI: 2.30 – 3.80), and invasive ICP monitoring (adjusted OR: 3.09, 95% CI: 2.28 – 4.16) were significantly associated with AKI (Table 4).In the ROC curve analysis associated with the prediction of AKI, there was no significant difference between the AUC of maximal measured osmolality (C-statistic: 0.646, 95% CI: 0.430 – 0.829) and that of the maximal osmolar gap (C-statistic: 0.633, 95% CI: 0.581 – 0.630) (p = 0.396) (Fig. 1).
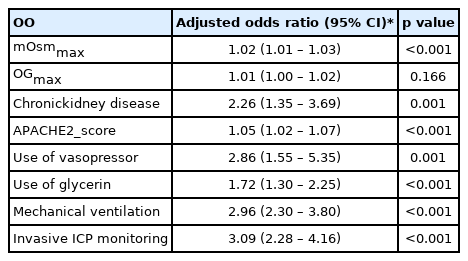
Multivariable logistic regression of clinically relevant variables associated with acute kidney injury

Receiver operating characteristic curves for prediction of poor outcomes using maximal measured osmolality (mOsmmax) and maximal osmolar gap (OGmax). The area under the curve of the mOsmmax (C-statistic: 0.646, 95% confidence interval [CI]: 0.430–0.829) was not statistically different with that of the OGmax (C-statistic: 0.633, 95% CI: 0.581–0.630) (p=0.396).
DISCUSSION
In this study, we investigated the clinical factors related to AKI during mannitol infusion and the impact of AKI on in-hospital mortality of neurosurgical patients and neurocritically ill patients. AKI was detected in 13.6% of all the patients on mannitol infusion, and the measured osmolarities and OG were significantly higher in patients with AKI than those without. Notably, the predictive power of the two indicators was similar, and both were weak predictors of AKI. In the multivariable analysis, maximal measured osmolality, chronic kidney disease, APACHE II score on ICU admission, use of vasopressor, use of glycerin, mechanical ventilation, and invasive ICP monitoring were significantly associated with AKI. Finally, in-hospital mortality and ICU mortality were significantly higher in patients with AKI than those without AKI.
Several reports have demonstrated that high doses and long-term usage of mannitol could easily result in kidney injury1) and therefore, it is necessary to monitor the level of serum mannitol during hyperosmolar therapy. However, while serum osmolality and OG are commonly used to monitor mannitol toxicity and to prevent AKI2), there is no routine monitoring protocol for serum level of mannitol. Mannitol infusion may be skipped or stopped with high serum osmolality and OG. In general, it is known that OG is a superior predictor of AKI2) than serum osmolality. On contrary, the results of the present study showed that the predictive power of the two indicators is similar, and both are weak predictors of AKI. Clinical factors related to ICU management and pre-existing kidney injury were associated with AKI than serum osmolarity and OG. Therefore, monitoring of serum osmolality and OG may still be important, but clinical factors and pre-existing renal disease may be more important in predicting AKI in the management of neurocritically ill patients.
In the previous study, AKI was a common and severe complication in the medical and surgical ICUs with high morbidity and mortality 12). Furthermore, AKI may be associated with increased costs and prolonged length of stay in the ICU and hospital12). AKI has also proven to be a major cause of poor clinical outcomes in ischemic stroke, subarachnoid hemorrhage, and traumatic brain injury patients 12-15). Moreover, mortality increased in proportion to the severity of AKI in neurocritically ill patients, and sepsis and chronic kidney disease were associated with AKI in neurocritically ill patients12) Similarly, in this study, the clinical outcomes, clinical factors related to ICU management and CKD were also associated with AKI in neurocritically ill patients.
This study has several limitations. First, this was a retrospective review of medical records using data extracted from a Clinical Data Warehouse. The nonrandomized nature of the registry data might have resulted in a selection bias. Second, while long-term and high doses of mannitol infusion may cause AKI, but the total amount of infused mannitol was factored in the analysis. Third, mild AKI was more common than moderate and severe AKI in this study. Finally, the distribution of neurosurgical diseases in the postoperative group was different from that of the general neurosurgical ICU group, and the proportion of patients with brain tumors was particularly high.
CONCLUSION
The results of the present study showed that kidney injury is associated with poor clinical outcomes in neurosurgical and neurocritically ill patients, and monitoring serum osmolality and OG remains important in preventing kidney injury in patients on mannitol infusion. Moreover, clinical factors related to ICU management and pre-existing renal disease were shown to aggravate AKI. Therefore, during mannitol infusion in neurosurgical and neurocritically ill patients, careful monitoring of laboratory tests and clinical factors related to ICU management can reduce kidney injury.
Notes
Conflict of interest
No potential conflict of interest relevant to this article was reported.
Acknowledgements
We would like to thank Hye Jung Kim, the nursing director of the neurosurgical intensive care unit, for providing excellent advice and engaging in fruitful discussions. We would also like to thank all nurses of the neurosurgery intensive care unit at the Samsung Medical Center.