 |
 |
- Search
| J Neurointensive Care > Volume 5(1); 2022 > Article |
|
Abstract
Objective
Patients with spinal cord injury (SCI) are often unstable and require intensive care unit (ICU) treatment in the acute phase. This study assists in the prognosis and treatment direction of SCI patients by retrospectively examining and analyzing the clinical characteristics of SCI patients admitted to the ICU.
Methods
In this study, a total of 102 SCI patients were admitted to the ICU of our hospital from February 2013 to March 2019. Based on the medical records, the patient's gender, age, mechanism of injuries, day of hospitalization, surgery timing, tracheostomy, ventilator use, steroid use, underlying disease, and hypotension were investigated. To assess the clinical outcome, the american spinal injury association (ASIA) impairment scale and limb motor grade three weeks and six months after injury was evaluated.
Results
Of the 102 patients, 76 (74.5%) were male, and the average age was 57.57 years. Of these, 87 (85.3%) had spinal surgery, while 30 (34.5%) had surgery within 36 h after injury. High doses of steroids were administered in 15 patients (14.9%). As for the ASIA impairment scale, Grade A at the initial stage of injury reached 15% of all patients but decreased to 5.1% after three weeks and 1.9% after six months.
Conclusion
Early surgery was advantageous in improving the lower extremity motor grade at three weeks of injury. Although steroid use has no significant effect compared to the risk of complications in several previous studies, in this study, it was observed that the ASIA scale improved six months after injury in patients receiving high-dose steroids.
Patients with severe spinal cord injury (SCI), including complete cervical spine injuries, are highly unstable in the acute phase and require hospitalization in the intensive care unit (ICU) with continuous cardiac, hemodynamic and respiratory monitoring1,2). Respiratory complications can quickly worsen within five days of injury, so active respiratory management should be initiated3). Hemodynamic instability should also be corrected quickly because recent studies recommended preventing systemic hypotension because maintaining proper spinal cord perfusion in patients with acute SCI may reduce long-term disability1,4). Additionally, SCI patients are vulnerable to hospital-acquired infections because they spend a considerable amount of time in the hospital5). Patients with traumatic spinal injuries also accompany other polytrauma injuries6), and the inability of self-hygiene due to paralysis, reflex loss, bedsores, tracheal intubation, prolonged placement of the catheter, and steroid use makes SCI patients susceptible to different infections, making the treatment of the disease more complex and challenging7).
Methylprednisolone has been noted for its potential as a therapeutic agent for SCI due to its inhibitory effect on active oxygen-induced lipid peroxidation, an essential biochemical mechanism of early SCI8). High-dose methylprednisolone has been widely used as an main medical treatment by many spine surgeons from the 1990s to the early 2000s since the National Acute Spinal Cord Injury Studies (NASCIS)9,10). However, in several randomized clinical studies afterward, it was reported that neurological improvement was insignificantly compared to the serious side effects1,8). It was suggested that the 2013 AANA/CNS guideline announced that steroid administration is no longer recommended4). Conversely, the positive effect of steroid administration is still reported sporadically, and the 2017 AOSpine guideline recommended it as a treatment option for acute SCI within eight hours of injury11).
Early decompression of the spinal cord helps improve clinical outcomes. In a meta-analysis of clinical trials, if the duration of spinal cord compression is long, studies are showing worsened results, including neurological recovery and blood circulation disorders12). However, until recently, clinical evidence is lacking, and there are no international guidelines for the timing of surgery1,13).
Since SCI leaves severe disability in patients, several studies have been conducted on the treatment of SCI. However, it is challenging to consistently control parameters in clinical studies of SCI patients due to various trauma mechanisms, degrees of cord injury, and types of spinal surgery. In the case of a prospective study, there is also an ethical problem in randomly selecting the treatment option for patients with critical cord injury rather than the best treatment considered by the attending physician.
To relatively standardize SCI patients, patients with traumatic cervical spine injuries were confined. Among them, it was assumed that patients who received ICU treatment were critically injured patients. To find the prognostic factors in patients with SCI, a retrospective study of these ICU patients was performed.
Traumatic cervical SCI patients treated at our hospital's ICU from February 2013 to May 2019 were retrospectively reviewed. Of the 123 traumatic SCI patients, 102 patients were included, excluding patients who were transferred to the general ward within 24 h of admission to the ICU and patients without adequate clinical data six months after the injury. Demographic data, spinal surgery, timing of surgery, tracheostomy, ventilator, steroid use, diabetes, infection, and hypotension of less than 90 mean arterial pressure within a week after injury were investigated using medical records. For clinical outcome analysis, upper and lower extremity muscle strength and ASIA impairment scale were analyzed at initial, three weeks and six months after the injury.
To discover the difference between the patient groups according to the surgery or not and the effect of surgery on the prognosis of patients, the patients were divided into two groups: the patient group who underwent surgery and the patient who did not undergo surgery. The group that underwent surgery was divided into a group that operated within 36 h after injury and a group that operated after 36 h for comparative analysis.
Categorical variables were analyzed with chi-square and FisherŌĆÖs exact tests, and continuous variables were compared with StudentŌĆÖs t-tests and MannŌĆōWhitney test. Multivariable logistic regression models were later made to control the potential compounding variables. Statistical calculations were performed using SPSS version 20.0 (IBM Corp., Armonk, NY, USA).
The patient demographics are listed in Table 1. Among the 102 patients, 76 were male (74.5%), and 26 were female (25.5%), and the average age was 57.57 years. The average total length of hospital stay was 65.04 d, and the average length of ICU stay was 13.28 d. Eighty-seven patients (85.3%) underwent spine-related surgery, and among them, 30 patients received operation within 36 h after injury. Seventy-seven (75.5%) were with the chief complaint of trauma, while nine (8.8%) of them were accompanied by skull fracture or traumatic intracranial hemorrhage. Ten patients (9.80%) required ventilator care during the ICU stay, and four patients (3.9%) required tracheostomy. Of these, 3 patients had failed extubation for more than 3 weeks, and the remaining 1 patient was an emergency tracheotomy due to a hematoma in the anterior cervical surgical site. The steroid was administered to 15 patients (14.9%) whereas sixteen patients (15.7%) had a comorbidity of diabetes mellitus. A total of 66 (64.7%) patients required consultation with the division of infectious diseases due to infectious diseases. Meanwhile, during ICU treatment, 91 (89.2%) patients had a mean arterial pressure (MAP) of less than 90.
Table 2 shows the results of comparative analysis of 102 enrolled patients divided into two groups, 87 who underwent surgery and 15 who did not. There were no differences in sex, mean age, head trauma, tracheostomy, mechanical ventilation, steroid use, diabetes, and hypotension between the groups. On the other hand, the total number of hospitalization and ICU treatment days tended to be longer in the non-surgical group but did not reach statistical significance. However, in the case of arm motor, the surgical group was significantly better at three weeks (p=0.029), but there was no difference around six months. In the case of leg motor, the surgical group exhibited significantly better results at six months (p=0.043).
The 87 patients who underwent surgery were compared by dividing the surgical timing into two groups of 36 h from injury (Table 3). Among the total surgery patients, 30 patients (34.5%) had surgery within 36 hours, and their average age was 50.7 years, which is about 10 years younger than the late surgery group. (p=0.009). Additionally, the total hospital stay and intensive care unit treatment days were longer in the early surgery group (p=0.068 and p=0.021, respectively), and steroid use was also significantly higher (p<0.001).
To analyze the factors related to the patient's prognosis, logistic regression analysis of the parameters for neurological symptoms 3 weeks and 6 months after SCI was performed (Table 4,-6). Logistic regression analysis showed that surgery within 36 hours had a significant positive correlation with improvement in leg motor after 3 weeks of SCI (Table 4), whereas steroid use showed a significant positive correlation with improvement of leg motor after 6 months. (Table 5). Although not presented, no significant parameters were observed in the regression analysis in the case of arm motor.
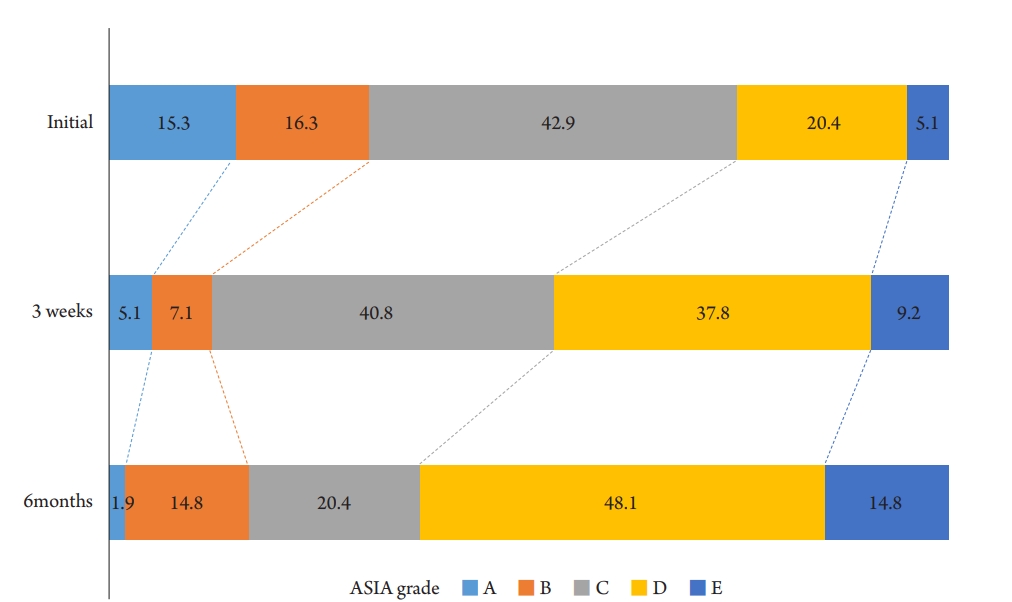
A graph comparing changes in the distribution of ASIA grade by period is indicated in Fig. 1. Initially, 42.9% of the patients were ASIA-C grade, and the most severe ASIA-A grade was 15.3%. However, after three weeks, the number of ASIA-A patients reduced to 5.1%, and the relatively mild cases of ASIA-D and E increased from 25.6%ŌĆō47.0%. At six months, 1.9% of all patients were still ASIA-A grade without improvement, and patients with ASIA-D and E accounted for 62.9%, which is 2.5 times higher than the initial. A regression analysis was also conducted on the correlation between the parameters and the degree of improvement of the ASIA grade, and steroid use was significant in the degree of improvement at six months (Table 6).
In this series, 66 patients (64.7%) developed a fever during hospitalization. Of these, 63 patients were treated with antibiotics, and the remaining three were atelectasis, steroid-related, and drug fever. The sites of infection and causative microorganisms of 63 patients (22 patients had more than one type of infection) with infection are indicated in Table 7. Regarding the occurrence of infection, urinary tract infection was the most common with 35 patients (57.5%), followed by respiratory tract infection 30 (49.2%) and skin infection. The microorganism was isolated 126 times; 50 (39.7%) were gram-positive, 67 (53.2%) were gram-negative, otherwise, eight (5.6%) were fungal and one (1%) was parasitic. Between the isolated microorganism of 126, Staphylococcus aureus (S.aureus) was isolated in 18 (14.3%). MRSA was isolated in 12 (9.5%), which occupied a lot among the isolated S.aureus. Bacteria producing extended-spectrum beta-lactamase were isolated in six (4.8%).
According to the study results that hypotension has adverse effects in the early stages of traumatic SCI, it was recommended to maintain the MAP at 85-90 in the first week as a recent guideline14). In 1976, Zach et al. conducted a prospective study of 117 patients with acute SCI in the ICU, which improved neurological outcomes through early, aggressive treatment and blood pressure management15). 1991 Wolf et al. In a study of 52 patients with bilateral cervical dislocation targeting a MAP > 85-mmHg for five days, they found that the patients exhibited neurological improvement.16) In 1993, Levi et al. (1993) targeted 50 SCI patients with MAP > 90-mm Hg more aggressively than MAP > 85-mmHg, and concluded that aggressive hemodynamic support reduced mortality and morbidity17). In 1997, Vale et al. reported that 77 SCI patients treated in the ICU with a MAP > 85-mmHg target and aggressive hemodynamic support for seven days indicated clinical improvement at one year follow-up18).
In patients with SCI, Spinal ischemia, which can be exacerbated by hypotension and hypoxia, plays a vital role in the occurrence of secondary progressive injury19). Intracellular mechanism of the SCI cascade, including neurogenic shock, microhemorrhage, ischemia-reperfusion injury, apoptosis, is related to secondary SCI20). Because of the fact that ischemia, hypotension and hypoxia can exacerbate secondary SCI, blood pressure control is one of the factors that play an essential role in reducing secondary SCI. Unfortunately, in patients with ACSI, hemodynamic and autonomic instability can often be found in the early stages of injury21). In this study, MAP of less than 90-mmHg was observed in 91 of 102 patients (89.2%) during the stay in ICU. Lower MAP than the guideline may have been caused by the underestimation of the importance of maintaining MAP. Additionally, the fact that autonomic nerve instability frequently appears in patients with severe SCI probably had an impact. Currently, our center maintains a MAP of 85 or higher in patients with severe SCI, even with vasopressors.
Respiratory complications in the acute phase of spinal cord injury are the leading cause of morbidity and mortality, accounting for 80.0% of mortality in patients with cervical SCI. Moreover, the prevalence of respiratory complications is very high, reaching 36.0%ŌĆō80.0%22). High mortality and morbidity in SCI patients can be explained by respiratory dysfunction due to damage of the respiratory muscles, decreased vital capacity, difficulty in sputum discharge, and decreased compliance of lung and chest wall23). And some of these patients require a ventilator, and long-term use of the ventilator will require tracheostomy23).
Tracheostomy rates in CSCI patients vary from 20.0% to 75.0%24). Although there is no clear standard for the timing of tracheostomy, some authors have argued that early tracheostomy (within 4 days) can reduce the duration of ICU hospitalization and respiratory complications, regardless of the degree of cervical spine injury25). Since we did not perform tracheostomy as it could interfere with the anterior cervical approach, the proportion of tracheostomy among all SCI patients was as low as 3.9%. We try extubation when the patient shows improvement in respiratory function, and tracheostomy is performed only for patients who have repeatedly failed extubation.
Patients with SCI are at high risk of healthcare-associated infections due to the frequent use of invasive medical devices, such as urinary and endovascular catheters. However, data on infection prevention and treatment in patients with SCI are lacking.26) Respiratory infections in patients with SCI are the most common during initial hospitalization, with a frequency of 60%. Streptococcus pneumoniae was the most common causative agent, and the prevalence of Pseudomonas was also observed to be high. Similarly, our study showed that Pseudomonas is the most prevalent cause of respiratory infection. Additionally, in patients with SCI, the typical pneumonia symptom occurrence rate is relatively low, making it challenging to detect pneumonia immediately. In our study, respiratory tract infection occurred in 30 patients (49.2%), and ten (9.8%) received ventilator treatment. Four patients (3.9%) underwent tracheostomy. So, it is crucial to ensure adequate preventive measures, such as vaccination against pneumonia26).
Urinary tract infection (UTI) is reported to develop within the first 50 d in 22% of patients with acute SCI, and is a significant cause of patient mortality and morbidity27). It is associated with urinary catheterization, and E. coli is the most common causative organism, which is the same as the results of this study. Even though the prevalence of UTI is high, the prevention method is unclear26). Considering the high prevalence of UTI in patients with SCI, periodic urine culture is suggested even when asymptomatic.
Bedsores also frequently act as a cause of infection in SCI patients. As a result of the bacteriological study of infected bedsores conducted by Heymet et al., the most frequently identified bacteria were Enterobacteriaceae group, accounting for 29%, and Staphylococcus spp. It was followed by 28% (mostly S. aureus)28). In our series, although relatively few compared to the previous literature, bedsore-related infections occurred in 14.8% of patients, mostly due to Staphylococcus aureus. Rapid identification and treatment of severe infections within the first 24 h after onset with appropriate antibiotics is associated with better outcomes. However, nearly 40% of SCI patients do not get adequate initial empirical antibiotic therapy26). In order to quickly identify an infection, we immediately and simultaneously perform blood, sputum, and urine cultures when a fever occurs. And before the causative organism is identified, empirical antibiotics are administered early through consultation with the Division of Infectious Diseases.
The American Spinal Injury Association (ASIA) Impairment Scale is commonly used to classify the neurological severity of SCI29), and is also being used because it is essential to view the prognosis of patients with SCI accurately30). In 2009, Martina R et al. conducted a cohort study in which SCI patients were classified using ASIA grade within two weeks of injury and at 1, 3, 6, and 12 months after injury. Of SCI patients classified as ASIA -A within 15 d of injury, 71.7% were still A at six months post-injury, 16.2% had ASIA-B, 4.7% had ASIA-C, 6.8% had ASIA-D, and 0.5% had converted to ASIA-E., And only 25% of patients initially analyzed using ASIA-B remained B, and most converted to C and D. More than 70% of ASIA-C converted to D, while nearly 90% of ASIA-D did not29). In recent years, it has been reported that the ASIA Impairement Scale conversion rate of patients with complete SCI has increased compared to the past30), which is thought to be due to the influence of the management of mean arterial blood pressure and the development of surgical techniques in managing traumatic SCI. In this study, the number of patients who had ASIA-A in the initial evaluation decreased from 15% to 1.9% at 6 months after injury, showing a significantly high ASIA Impairment Scale conversion rate.
Components of secondary SCI, such as neuropathy, microhemorrhage, ischemia-reperfusion injury, and apoptosis, are major targets for prophylactic intervention and treatment in patients with acute SCI31). Although methylprednisolone had been spotlighted as a treatment regimen to prevent this secondary SCI, In NASCIS II and III, which were adopted by organizations worldwide, there is no proven evidence that methylprednisolone makes neurological improvement8,32). In addition, some studies have pointed out that high-dose steroid use in patients with acute spinal cord injury is skeptical because of the risk of serious side effects compared to the neurological benefits8). On the other hand, some studies have still shown benefits from the use of high-dose methylprednisolone33). To date, there is no clear alternative to steroids to the preventive intervention of secondary SCI. Even the slight improvement in sphincter control or finger motor function reported in NASICS trials can have a decisive effect on a patient's life. Therefore, some argue that it may be ethically justified to use drugs with potential efficacy in patients with SCI20). In our department, high-dose steroid therapy is not used in elderly patients. This is because there is currently insufficient evidence to support the benefits of steroid use versus side effects. However, in a young, severe SCI patients, methylprednisolone 500-mg/day was started, and the dose was gradually reduced over seven days. Methylprednisolone-treated patients showed improvement in lower extremity muscle strength and ASIA grade 6 months after injury, and there was one serious side effect along with fever due to leukemoid reaction. However, caution is needed in the interpretation of the efficacy of methylprednisolone use in this retrospective study because the steroid treatment group was characterized by relatively young age and severe injuries.
Many spine surgeons have agreed on the benefits of early surgery in SCI patients with spinal cord compression or spinal instability. However, there is no consensus on the most favorable time for surgery, as it varies from study to study, such as within 36 hours or within 72 hours34-37). In a prospective cohort study of 313 patients with acute SCI, Fehlings et al. found that the group that underwent surgery at a mean of 14 hours showed at least a grade 2 improvement in the ASIA injury scale after 6 months compared to the group that underwent surgery at a mean of 48 hours35). And Haldrup et al suggested that patients who underwent surgery within 24 hours had better neurological outcomes 1 year after trauma than those who underwent surgery after 24 hours36). In this study, 30 patients (34.48%) who underwent surgery within 36 hours showed relatively greater improvement in lower extremity strength 3 weeks after injury. Early surgical treatment shortens the ICU hospital stay, reduces complications, and improves neurological outcomes, so we suggest that early surgery is recommended except for patients who are difficult to operate due to unstable vital signs.
The comorbidity of traumatic brain injury (TBI) is reported in 16%-59%38), and this comorbidity is an important problem that increases the probability of early death in patients with spinal cord injury by 370%39). In this study, 66 (64.7%) patients had a head impact and underwent an initial brain CT scan. Of these, nine (13.6%) patients with contusion, hemorrhage with or without skull fracture were observed using a CT scan. TBI's severity may interfere with the reliability of the initial neurological examination, which is vital as it may interfere with patient treatment planning and outcome prediction. High conversion rates can be shown in early hours or days after injury in patients with complete SCI who are misclassified due to inaccurate initial neurological examination40).
This study has some drawbacks because it was a retrospective study conducted at a single institution. There were limits to the control of various variables that could affect the prognosis, such as the extent and severity of SCI, the mechanism of trauma, and the underlying diseases. In addition, a bias is expected because steroid administration subjects were not randomly selected, but relatively young patients with few underlying diseases were selected. However, we tried to reduce the heterogeneity of patients by limiting the study subject to SCI patients who require treatment in the ICU. Short-term and long-term prognosis were analyzed with various risk factors. In addition, the prevalence of infection sites and microorganisms in SCI patients was thoroughly investigated. These data are expected to provide useful information for the treatment of SCI. And although caution is required in interpretation, the findings that steroid use and early surgery improved the prognosis of patients with spinal cord injury suggest that further studies on these treatments are still needed.
SCI causes different fatal complications, and treatment for these complications is critical in preventing secondary damage to the spinal cord and decreasing morbidity in patients. In this study, early surgery within 36 hours of injury was meaningful in improving lower extremity motor grade after 3 weeks in SCI patients, and it was observed that the ASIA injury scale was further improved at 6 months of injury in patients receiving high-dose steroids.
Fig.┬Ā1.
A graph comparing changes in the distribution of american spinal injury association (ASIA) grade by period.
Table┬Ā1.
Patients demographics
Table┬Ā2.
Comparison between the surgery group and the non-surgery group
| Group | Surgery (N=87) | Non-surgery (N=15) | p value |
|---|---|---|---|
| Male | 66 (75.9%) | 10 (66.7%) | 0.450 |
| Age | 57.17 ┬▒ 16.91 | 59.87 ┬▒ 17.30 | 0.583 |
| Hospitalization days | 66.95 ┬▒ 59.14 | 53.93 ┬▒ 62.49 | 0.067ŌĆĀ |
| ICU.day | 11.90 ┬▒ 28.40 | 21.33 ┬▒ 33.80 | 0.364ŌĆĀ |
| Head trauma | 8 (9.2%) | 1 (6.7%) | 1.000 |
| Tracheostomy | 4 (4.6%) | 0 (0.0%) | 1.000 |
| Mechanical ventilation | 8 (9.2%) | 2 (13.3%) | 0.639 |
| Arm motor improvement | |||
| ŌĆā3 weeks | 0.64 ┬▒ 1.43 | 0.25 ┬▒ 0.50 | 0.029*,ŌĆĀ |
| ŌĆā6 months | 1.22 ┬▒ 1.66 | 1.00 ┬▒ 2.16 | 0.985* |
| Leg motor improvement | |||
| ŌĆā3 weeks | 0.80 ┬▒ 1.22 | 0.25 ┬▒ 0.50 | 0.120 |
| ŌĆā6 months | 1.49 ┬▒ 1.58 | 0.25 ┬▒ 1.26 | 0.043*,ŌĆĀ |
| Steroid use | 12 (13.8%) | 3 (20.0%) | 0.433 |
| Diabetes | 13 (14.9%) | 3 (20.0%) | 0.701 |
| Hypotension | 78 (89.7%) | 13 (86.7%) | 0.663 |
Table┬Ā3.
Comparison between the groups according to the surgery timing
| Group | Surgery < 36h (N=30) | Surgery Ōēź 36h (N=57) | p value |
|---|---|---|---|
| Male | 25 (83.3%) | 41 (71.9%) | 0.359 |
| Age | 50.70 ┬▒ 15.34 | 60.58 ┬▒ 16.83 | 0.009* |
| Hospitalization days | 85.90 ┬▒ 80.20 | 56.98 ┬▒ 41.80 | 0.068ŌĆĀ |
| ICU.day | 20.17 ┬▒ 45.19 | 7.54 ┬▒ 11.19 | 0.021ŌĆĀ |
| Head trauma | 4 (13.3%) | 4 (7.0%) | 0.563 |
| Tracheostomy | 2 (6.7%) | 2 (3.5%) | 0.897 |
| Mechanical ventilation | 5 (16.7%) | 3 (5.3%) | 0.174 |
| Arm motor improvement | |||
| ŌĆā3 weeks | 1.14 ┬▒ 1.75 | 0.42 ┬▒ 1.23 | 0666ŌĆĀ |
| ŌĆā6 months | 1.50 ┬▒ 1.74 | 1.10 ┬▒ 1.64 | 0.496ŌĆĀ |
| Leg motor improvement | |||
| ŌĆā3 weeks | 1.07 ┬▒ 0.73 | 0.68 ┬▒ 1.38 | 0.210ŌĆĀ |
| ŌĆā6 months | 1.36 ┬▒ 1.34 | 1.55 ┬▒ 1.69 | 0.903 |
| Steroid use | 10 (33.3%) | 2 (3.5%) | < 0.001* |
| Diabetes | 3 (10.0%) | 10 (17.5%) | 0.534 |
| Hypotension | 26 (86.7%) | 52 (91.2%) | 0.769 |
Table┬Ā4.
Logistic regression analysis of leg motor improvement at three weeks
| Variable |
Univariate analysis |
Multivariate analysis |
||
|---|---|---|---|---|
| p value | Odds ratio | p value | Odds ratio | |
| Age | 0.811 | 1.004 | ||
| Surgery within 36 h | 0.007* | 6.448 | 0.006* | 6.369 |
| Steroid use | 0.075 | 0.215 | 0.073 | 0.226 |
| Hypotension | 0.487 | 0.577 | ||
| Diabetes | 0.696 | 0.767 | ||
Table┬Ā5.
Logistic regression analysis of leg motor improvement at six months
| Variable |
Univariate analysis |
Multivariate analysis |
||
|---|---|---|---|---|
| p value | Odds ratio | p value | Odds ratio | |
| Age | 0.797 | 0.993 | ||
| Surgery within 36h | 0.291 | 3.316 | ||
| Steroid use | 0.020* | 0.022 | 0.015* | 0.061 |
| Hypotension | 0.442 | 0.339 | ||
| Diabetes | 0.365 | 0.351 | ||
Table┬Ā6.
Logistic regression analysis of ASIA grade improvement at six months
| Variable | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| p value | Odds ratio | p value | Odds ratio | |
| Age | 0.583 | 0.987 | ||
| Surgery within 36h | 0.080 | 7.055 | 0.077 | 6992 |
| Steroid use | 0.037* | 0.045 | 0.038* | 0.063 |
| Hypotension | 0.283 | 0.271 | ||
| Diabetes | 0.919 | 0.906 | ||
Table┬Ā7.
The prevalence of infectious diseases in SCI patients
| Site of infectionb | Patient No. (%)a |
| Renal/Urinary tract | 35 (57.4%) |
| Respiratory tract | 30 (49.2%) |
| Skin | 9 (14.8%) |
| Abdominal | 3 (4.9%) |
| Central nervous systemc | 2 (3.3%) |
| Catheter related blood stream | 2 (3.3%) |
| Another sited | 3 (4.9%) |
| Microorganismsb | No. (%)a |
| Positive isolates | 126 (100%) |
| Gram-positive | 50 (39.7%) |
| ŌĆāMSSA | 6 (12.0%) |
| ŌĆāMRSA | 12 (24.0%) |
| ŌĆāStaphylococcus epidermidis | 4 (8.0%) |
| ŌĆāOther Staphylococcus speciese | 9 (18.0%) |
| ŌĆāVSE | 9 (18.0%) |
| ŌĆāVRE | 2 (4.0%) |
| ŌĆāStreptococcus pneumonia | 1 (2.0%) |
| ŌĆāOther Streptococcus speciesf | 1 (2.0%) |
| ŌĆāClostridioides difficile | 3 (6.0%) |
| ŌĆāOthersg | 3 (6.0%) |
| Gram-negative | 67 (53.2%) |
| ŌĆāESBL-producing Escherichia coli | 4 (6.0%) |
| ŌĆāNon-ESBL-producing Escherichia coli | 6 (9.0%) |
| ŌĆāPseudomonas species | 14 (20.9%) |
| ŌĆāAcinetobacter species | 20 (29.9%) |
| ŌĆāESBL-producing Klebsiella species | 2 (3.0%) |
| ŌĆāNon-ESBL-producing Klebsiella species | 10 (14.9%) |
| ŌĆāEnterobacter species | 2 (3.0%) |
| ŌĆāHaemophilus influenzae | 2 (3.0%) |
| ŌĆāOthersh | 7 (10.4%) |
| Fungi | 8 (6.3%) |
| ŌĆāCandida species | 8 (100%) |
| ŌĆāAspergillus species | 0 (0.0%) |
| ŌĆāOthers | 0 (0.0%) |
| Parasitesi | 1 (0.8%) |
SCI: Spinal cord injury, MSSA: Methicillin-susceptible Staphylococcus aureus, MRSA: Methicillin-resistant Staphylococcus aureus, VRE: vancomycin-resistant Enterococcus, VSE: Vancomycin-sensitive Enterococcus, ESBL: Extended-spectrum ╬▓-lactamases.
a Percentage do not necessarily equal 100 because 63 patients consulted with Infectious Medicine may have had more than 1 type of infection or microorganism.
e Including Staphylococcus capitis (7), Staphylococcus caprae (1), and Staphylococcus haemolyticus (1).
REFERENCES
1. Ahuja CS, Wilson JR, Nori S, Kotter MRN, Druschel C, Curt A, et al. Traumatic spinal cord injury. Nat Rev Dis Primers 2017;3:17018.


2. Resnick DK. Updated guidelines for the management of acute cervical spine and spinal cord injury. Neurosurgery 2013;72 Suppl 2:1.

3. Berlly M, Shem K. Respiratory management during the first five days after spinal cord injury. J Spinal Cord Med 2007;30:309ŌĆō318.



4. Walters BC, Hadley MN, Hurlbert RJ, Aarabi B, Dhall SS, Gelb DE, et al. Guidelines for the management of acute cervical spine and spinal cord injuries: 2013 update. Neurosurgery 2013;60:82ŌĆō91.


5. Montgomerie JZ. Infections in patients with spinal cord injuries. Clin Infect Dis 1997;25:1285ŌĆō1290; quiz 1291-1282.


6. Yue JK, Winkler EA, Rick JW, Deng H, Partow CP, Upadhyayula PS, et al. Update on critical care for acute spinal cord injury in the setting of polytrauma. Neurosurg Focus 2017;43:E19.


7. Sugarman B, Brown D, Musher D. Fever and infection in spinal cord injury patients. JAMA 1982;248:66ŌĆō70.


8. Hall ED, Springer JE. Neuroprotection and acute spinal cord injury: a reappraisal. Neurorx 2004;1:80ŌĆō100.



9. Bracken MB, Shepard MJ, Collins WF, Holford TR, Young W, Baskin DS, et al. A randomized, controlled trial of methylprednisolone or naloxone in the treatment of acute spinal-cord injury. Results of the second National Acute Spinal Cord Injury Study. N Engl J Med 1990;322:1405ŌĆō1411.


10. Bracken MB, Shepard MJ, Holford TR, Leo-Summers L, Aldrich EF, Fazl M, et al. Administration of methylprednisolone for 24 or 48 hours or tirilazad mesylate for 48 hours in the treatment of acute spinal cord injury. Results of the third National Acute Spinal Cord Injury randomized controlled trial. National Acute Spinal Cord Injury Study. JAMA 1997;277:1597ŌĆō1604.


11. Fehlings MG, Wilson JR, Tetreault LA, Aarabi B, Anderson P, Arnold PM, et al. A clinical practice guideline for the management of patients with acute spinal cord injury: recommendations on the use of methylprednisolone sodium succinate. Glob Spine J 2017;7 Suppl:203SŌĆō211S.

12. Batchelor PE, Wills TE, Skeers P, Battistuzzo CR, Macleod MR, Howells DW, et al. Meta-analysis of pre-clinical studies of early decompression in acute spinal cord injury: a battle of time and pressure. PLOS ONE 2013;8:e72659.



13. Fehlings MG, Rabin D, Sears W, Cadotte DW, Aarabi B. Current practice in the timing of surgical intervention in spinal cord injury. Spine (Phila Pa 1976) 2010;35 Suppl:S166ŌĆōS173.

14. Tee JW, Altaf F, Belanger L, Ailon T, Street J, Paquette S, et al. Mean arterial blood pressure management of acute traumatic spinal cord injured patients during the pre-hospital and early admission period. J Neurotrauma 2017;34:1271ŌĆō1277.


15. Z├żch GA, Seiler W, Dollfus P. Treatment results of spinal cord injuries in the Swiss Parplegic Centre of Basle. Paraplegia 1976;14:58ŌĆō65.


16. Wolf A, Levi L, Mirvis S, Ragheb J, Huhn S, Rigamonti D, et al. Operative management of bilateral facet dislocation. J Neurosurg 1991;75:883ŌĆō890.


17. Levi L, Wolf A, Belzberg H. Hemodynamic parameters in patients with acute cervical cord trauma: description, intervention, and prediction of outcome. Neurosurgery 1993;33:1007ŌĆō1016;discussion 1016-1007.


18. Vale FL, Burns J, Jackson AB, Hadley MN. Combined medical and surgical treatment after acute spinal cord injury: results of a prospective pilot study to assess the merits of aggressive medical resuscitation and blood pressure management. J Neurosurg 1997;87:239ŌĆō246.


19. Hawryluk G, Whetstone W, Saigal R, Ferguson A, Talbott J, Bresnahan J, et al. Mean arterial blood pressure correlates with neurological recovery after human spinal cord injury: analysis of high frequency physiologic data. J Neurotrauma 2015;32:1958ŌĆō1967.



20. Rozet I. Methylprednisolone in acute spinal cord injury: is there any other ethical choice? J Neurosurg Anesthesiol 2008;20:137ŌĆō139.

21. Lehmann KG, Lane JG, Piepmeier JM, Batsford WP. Cardiovascular abnormalities accompanying acute spinal cord injury in humans: incidence, time course and severity. J Am Coll Cardiol 1987;10:46ŌĆō52.


22. Tollefsen E, Fondenes O. Respiratory complications associated with spinal cord injury. Tidsskr Nor Laegeforen 2012;132:1111ŌĆō1114.



23. Brown R, DiMarco AF, Hoit JD, Garshick E. Respiratory dysfunction and management in spinal cord injury. Respir Care 2006;51:853ŌĆō868;discussion 869-870; .

24. Branco BC, Plurad D, Green DJ, Inaba K, Lam L, Cestero R, et al. Incidence and clinical predictors for tracheostomy after cervical spinal cord injury: a National Trauma Databank review. J Trauma 2011;70:111ŌĆō115.


25. Anand T, Hanna K, Kulvatunyou N, Zeeshan M, Ditillo M, Castanon L, et al. Time to tracheostomy impacts overall outcomes in patients with cervical spinal cord injury. J Trauma Acute Care Surg 2020;89:358ŌĆō364.


26. Garcia-Arguello LY, OŌĆÖHoro JC, Farrell A, Blakney R, Sohail MR, Evans CT, et al. Infections in the spinal cord-injured population: a systematic review. Spinal Cord 2017;55:526ŌĆō534.


27. Togan T, Azap OK, Durukan E, Arslan H. The prevalence, etiologic agents and risk factors for urinary tract infection among spinal cord injury patients. Jundishapur J Microbiol 2014;7:e8905.



28. Heym B, Rimareix F, Lortat-Jacob A, Nicolas-Chanoine MH. Bacteriological investigation of infected pressure ulcers in spinal cord-injured patients and impact on antibiotic therapy. Spinal Cord 2004;42:230ŌĆō234.


29. Spiess MR, M├╝ller RM, Rupp R, Schuld C, EM-SCI Study Group, van Hedel HJ. Conversion in ASIA impairment scale during the first year after traumatic spinal cord injury. J Neurotrauma 2009;26:2027ŌĆō2036.


30. Marino RJ, Leff M, Cardenas DD, Donovan J, Chen D, Kirshblum S, et al. Trends in rates of ASIA impairment scale conversion in traumatic complete spinal cord injury. Neurotrauma Rep. Neurotrauma Rep 2020;1:192ŌĆō200.



31. Canseco JA, Karamian BA, Bowles DR, Markowitz MP, DiMaria SL, Semenza NC, et al. Updated review: the steroid controversy for management of spinal cord injury. World Neurosurg 2021;150:1ŌĆō8.


32. Hurlbert RJ. Methylprednisolone for acute spinal cord injury: an inappropriate standard of care. J Neurosurg 2000;93 Suppl:1ŌĆō7.

33. Tsutsumi S, Ueta T, Shiba K, Yamamoto S, Takagishi K. Effects of the second National Acute Spinal Cord Injury Study of high-dose methylprednisolone therapy on acute cervical spinal cord injury-results in spinal injuries center. Spine (Phila Pa 1976) 2006;31:2992ŌĆō2996;discussion 2997.


34. Chipman JG, Deuser WE, Beilman GJ. Early surgery for thoracolumbar spine injuries decreases complications. J Trauma 2004;56:52ŌĆō57.


35. Harrop JS, et al. Early versus delayed decompression for traumatic cervical spinal cord injury: results of the Surgical Timing in Acute Spinal Cord Injury Study (STASCIS). PLOS ONE 2012;7:e32037.



36. Haldrup M, Schwartz OS, Kasch H, Rasmussen MM. Early decompressive surgery in patients with traumatic spinal cord injury improves neurological outcome. Acta Neurochir (Wien) 2019;161:2223ŌĆō2228.


37. Lubelski D, Tharin S, Como JJ, Steinmetz MP, Vallier H, Moore T. Surgical timing for cervical and upper thoracic injuries in patients with polytrauma. J Neurosurg Spine 2017;27:633ŌĆō637.


38. Macciocchi S, Seel RT, Thompson N, Byams R, Bowman B. Spinal cord injury and co-occurring traumatic brain injury: assessment and incidence. Arch Phys Med Rehabil 2008;89:1350ŌĆō1357.


39. Varma A, Hill EG, Nicholas J, Selassie A. Predictors of early mortality after traumatic spinal cord injury: a population-based study. Spine (Phila Pa 1976) 2010;35:778ŌĆō783.

40. Khorasanizadeh M, Yousefifard M, Eskian M, Lu Y, Chalangari M, Harrop JS, et al. Predictors of early mortality after traumatic spinal cord injury: a population-based study. J Neurosurg Spine 2019;1ŌĆō14.
- TOOLS
-
METRICS

-
- 0 Crossref
- 3,378 View
- 88 Download
- Related articles in JNIC
-
Neurological Intensive Care for Acute Spinal Cord Injury Patients2018 August;1(1)





