Clinical Outcomes of Cranioplasty Using a Customized Artificial Bone Flap Made by a 3D Printing Technique
Article information
Abstract
Objective
To analyze the outcomes of using a customized artificial skull bone flap made with a three-dimensional (3D) printing technique for cranioplasty and compare them with those of the autobone technique.
Method
Between December 2018 and February 2020, 24 cranioplasties were performed for 24 hemispheres using a customized artificial bone flap made by 3D printing. Simultaneously, 19 cranioplasties for 19 hemispheres were performed using an autobone. Three patients underwent cranioplasty using both a customized artificial bone flap and autobone for each hemisphere. Patient’s demographics, reason for craniectomy, interval between craniectomy and cranioplasty, surface area of the skull defect and bone flap, bone flap coverage of the defect, cranioplasty-related factors, and clinical outcome were assessed.
Results
Forty patients who underwent cranioplasty (bone flap, 21; autobone flap, 16; and artificial bone/autobone flaps, 3) were enrolled. The artificial bone flap covered more skull defects than the autobone flap (98.6% vs. 90.9%, p=0.000). There were two and six operation-related complications in the artificial bone flap and autobone flap groups, respectively (P=0.061). The subtemporal area was completely covered in the artificial bone flap group. Two patients had an infection of the autobone flap and had it replaced by a 3D printing flap. No patient showed a reduction in the modified Rankin Scale score after surgery, and the clinical course was confirmed to have improved.
Conclusion
Cranioplasty using customized artificial bone flap made by 3D printing technique was effective for covering the skull defect and tends to have a low complication rate compared to the autobone.
INTRODUCTION
Cranioplasty is required to restore the appearance of the skull, protect intracranial tissue, and improve neurological function1,2). Autologous bone flaps are usually the first choice at most institutions, as they are readily available at no additional cost and yield excellent outcomes3,4).
Although autologous bone remains the first choice for repair, it is not always used because of infection, fragmentation, bone resorption, or other causes5,6), which has led to the use of synthetic alternatives7). The ideal cranioplasty material is nonmagnetic, radiolucent, light, sterilizable, and easily affixed to the skull. Currently, the three most popular nonbiological materials used for this purpose are polymethylmethacrylate (PMMA), hydroxyapatite, and titanium meshes or plates. However, trials using materials such as polyether ether ketone and porous polyethylene are also being conducted8).The procedures for using these materials vary between centers, with some preferring to shape the implant intraoperatively, while others choose to create custom-made plates preoperatively using computed tomography (CT) or stereolithography data8,9,10).
Three-dimensional (3D) printing techniques are evolving, and their application in medical technology, such as utilizing it to make artificial bone flaps for various surgeries is increasing,11,12). This study aimed to analyze the outcomes of using a customized artificial skull bone flap made by a 3D printing technique for cranioplasty, compared with autobone. Significant results were confirmed as statistical differences between the results of cases.
MATERIALS AND METHODS
This study was a retrospective study of medical records, including a survey of satisfaction after 3D cranioplasty (3DC). All procedures performed in studies involving human participants were in accordance with the ethical standards of the Ethical Committee of Busan Paik Hospital of Inje Medical University and with the Helsinki Declaration. Since this study was conducted retrospectively, it was exempted from that criterion.
Patient data
The data of 40 patients who underwent cranioplasty between December 2018 and February 2020 were included in the study. All patients in this study were included in accordance with criteria. No patients were excluded. A total of 24 cranioplasties for 24 hemispheres using a customized artificial bone flap made by 3D printing technique were performed. Simultaneously, 19 cranioplasties were performed for 19 hemispheres using autobone. Among them, three patients underwent cranioplasty using both customized artificial bone flap and autobone for each hemisphere.
We assessed the patients’ demographics, reason for craniectomy, interval between craniectomy and cranioplasty, operation time, bleeding volume, surface area of the skull defect and bone flap, bone flap coverage of the defect, cranioplasty-related factor, comparison between the two groups, and 6-month clinical outcome including telephone survey of satisfaction after 3DC.
Cranioplasty style and material
All customized artificial 3D printing bone flaps were made by PRYMARY. (CUSMEDI, South Korea), which is a patient-specific implant using titanium alloy (Ti6Al4V ELI in accordance with ASTM F136&F3001-14) produced via selective laser melting additive manufacturing/3D printing. Preoperative CT scan data (1 mm slice thickness) were converted to 3D images (Aview Modeler, CORELINE, South Korea), and the implant was designed using a mirror-imaging process of the normal site of the cranium using Materialize Magics (3D Systems, USA). The surface of the 3D implant model was processed using a smoothing technique by the Meshmixer (Ver. 3.5, Autodesk, United States of America).
The operation procedure was as follows. Under general endotracheal anesthesia, the patient was placed on the operating table in the supine position and the head was positioned. The whole scalp was prepared with scrub-povidone, povidone, and draped. A scalp incision was made along the previous surgical scar, and bleeding was controlled with a bovi and bipolar coagulator. The scalp flap was reflected, and the adhesied part of the scalp was adhesiolized with a No. 15 blade and bovi monopolar coagulator. Bleeding from the inner surface of the scalp and muscle was controlled with a bipolar coagulator. The soft tissue overlying the dura was separated from the dura. The 3D printing flap was fixed with screws, and the autobone flap was fixed with miniplates and screws. After central tagging and meticulous bleeding control, the wound was closed in layers. The patients tolerated the procedure described above. (Fig. 1)
Measurement of craniectomy lesions and defects
In the case of 3DC, the defect values and flap surface area were measured using Mimics Research Version 19.0 and 3-matic Research 11.0 made by Materialize. (Leuven, Belgium) After obtaining the craniectomy area and autobone flap area using Materialise Mimics and 3-matic, respectively, the subtraced area was defined as the defect area. The average circumference of the craniectomy area and autobone flap was calculated, and the average defect width was calculated using the formula described below.
Fig. 2 illustrates the defects of craniectomy and bone flap and the parameters of the formula.
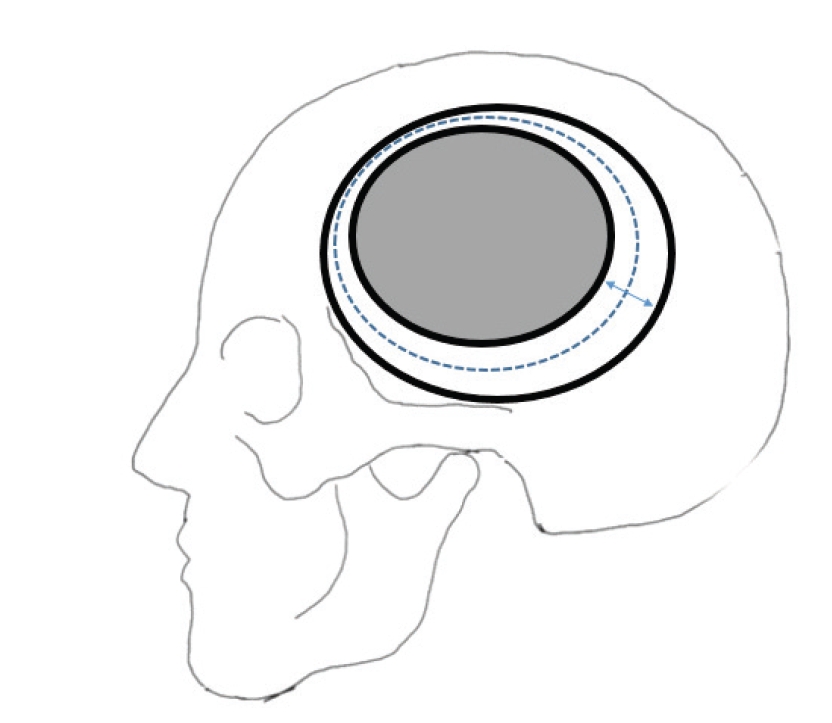
The formula of a skull defect, bone flap, and parameters of the formula. The external bold-lined oval is a skull defect, and the internal bold-lined oval is a bone flap. The dotted lined oval between two bold-lined ovals is the average length of the circumstance of the bone flap and skull defect. The bidirectional arrow indicates the average of the defect in cranioplasty.
Statistical analysis
Statistical analyses were performed using SPSS for Windows (version 26; SPSS, Chicago, IL, USA). Univariate analysis was performed with the time interval between craniectomy and cranioplasty, hemisphere (right/left/both), operation time, blood loss, complications, circumstance of the autobone flap, circumstance of craniectomy, surface area of the skull defect, surface area of the bone flap, bone-flap coverage of the skull defect, area of defect (mm2), and average of defect (mm) as factors using the Student’s t-test, chi-square test, or Fisher’s exact test due to the expected frequency.
RESULTS
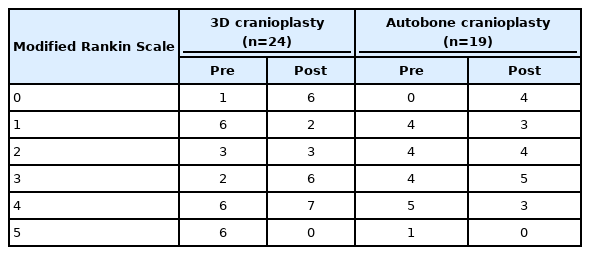
Forty patients were included in this study (average age, 51.1 years; 21 male). 3DC was performed in 24 patients (average age, 49.5 years; 16 male) and autobone cranioplasty (AC) was performed in 19 patients (average age 49.7 years; 8 male). Among them, 26 patients had cerebrovascular accidents (cerebral infarction, aneurysmal spontaneous subarachnoid hemorrhage, spontaneous intracranial hemorrhage (ICH), one brain abscess, and 13 head traumas. The mean interval between craniectomy and cranioplasty was 5.6 months (3DC group, 6.6 months; AC group, 4.3 months; p=0.123). The affected hemispheres were 27 on the right and 17 on the left (p=0.096; in one case, AC for both frontal craniectomies were performed in one step. The average operation time was 167.3 min (3DC group, 168.5 min; AC group, 165.8 min; p=0.889). The average blood loss during operations was 172 mL (3DC group, 184 mL; AC group, 156 156 mL; p=0456). Cranioplasty-related complications occurred in eight cases (3DC group, two; AC group, six; p=0.061). There were no statistically significant differences in age, sex, interval between craniectomy and cranioplasty, operation time, and blood loss between the two groups. In four patients in the AC group, cranioplasty was accompanied by bone cement. (Table 1)
The average of circumference of craniectomy was 403 mm (3DC group, 399 mm; AC group, 408 mm; p=0.730) and surface area of skull defect was 103.93 cm2 (3DC, 106.74 cm2; AC, 100.39 cm2; p=0.503). The average bone flap circumference was 363 mm. (3DC, 366 mm; AC, 359 mm; p=0.687), and average of surface area of the bone flap was 98.95 cm2 (3DC, 105 cm2, AC, 90 cm2; p=0.112). However, bone flap coverage of the skull defect was 95.2% (3DC, 98.6%, AC, 90.9%; p=0.000), and the area of defect was 498 mm2 (3DC, 146 mm2, AC, 943 mm2; P-value 0.000). In addition, the average size of defect was 1.23 mm (3DC, 0.35 mm, AC, 2.35 mm; p=0.000). In this data, bone flap coverage, area of defect, and average of defect showed statistically significant differences (p< 0.000), and are summarized in Table 2. Supplementary Fig. 1 shows the high skull defect coverage of the 3DC.
In the case of cranioplasty-related complications, there were two operation-related complications (one case of cosmetic issues and one case of ICH after cranioplasty) in the 3DC group, and six (one episode of extradural hematoma after cranioplasty, one case of cosmetic issue, two cases of bone floating, and two cases of infection requiring bone-flap removal) in the AC group. (p=0.061) Table 3 summarizes the data. Temporal muscle atrophy was inevitable even with 3DC. (Supplementary Fig. 2) Regarding AC, bone flap floating occurred in two cases. (Fig. 3)

Temporal bone floating after autobone cranioplasty. This may have occurred because of incomplete temporal muscle dissection.
In the 3DC group, the preoperative modified Rankin Scale (mRS) scores were as follows: (4 points, 6 patients; 5 points, 6 patients). The postoperative scores were as follows: (4 points, 7 patients; 5 points, 0 patients). In addition, preoperative mRS scores of the AC group were as follows: (4 points, 5 patients; 5 points, 1 patient). The postoperative scores were as follows: (4 point, 3 patients; 5 point, 0 patients). There was no patient with a lowering of mRS after the operation in any group, and the clinical course was confirmed to have improved. (Table 4)
DISCUSSION
A large format (i.e., > 25 cm2) cranioplasty is a challenging procedure not only from a cosmetic standpoint, but also in terms of ensuring that the patient's brain is well protected from direct trauma, sinking flap syndrome, and syndrome of the trephined. Until recently, when a patient's cranial flap was unavailable, it was difficult to achieve these goals. It is now possible to 3-D-print patient-specific implants from a variety of polymer, ceramic, or metal components. A 3D printing design may be used to imitate the previous external shape of the skull that will become well integrated into the skull while providing beneficial distribution of mechanical force in the event of trauma13). Recently, 3D printing flaps have been increasingly used in patients undergoing cranioplasty14,15).
If 3D printing flaps are used, there would be advantages in the infection ratio and cosmetic area16). In this study, we evaluated and compared the defects between the autobone flap and 3D printing flap for cosmetic points. In general, craniectomy is focused on the temporal area to achieve maximal decompression. With the increase in subtemporal decompression, the prognosis of the patient would improve if the shape of the midbrain is restored and the cerebrospinal fluid flow is smooth17). However, the removed skull bone can remain as temporal bone defects that are not well covered in AC. In the case of the 3D printing flaps, the temporal defect can be solved by imitating the unaffected skull.
There are multiple holes in the 3D printing flap. Using these multiple holes, sufficient dural tenting can be performed to reduce additional manipulation. When using an autobone, a suture hole for tenting must be made using a drill, hand manipulation is involved, and the number of holes is limited. In a 3D printing flap, dural tenting can be performed more easily because of multiple holes. However, epidural fluid collection occurred in both groups in this study. Such complications vary depending on the surgeon. There was a difference in the dissection of the adhesion site and epidural space control. To prevent epidural fluid collection, it is important to meticulously check the leakage point and perform duroplasty. In the case of autobone complications that were not seen in 3D printing cranioplasty, calcified deposits were found in the soft tissue. In the case of AC, cement is used as a complementary method for the defect. Forming cement for cranioplasty can increase the infection risk by using hand manipulation and develop complications in which a fragment of cement is found in soft tissue, as in the case of a patient in this study.
Additional treatment caused by a cranioplasty defect may increase the risk of infection due to an increase in operation time and hand manipulation. However, in the case of 3DC, all defects were designed to be covered, and this might have been better than the autobone flap in this regard. Postoperative infection was not observed in the 3DC group. If an infection develops after AC, it is impossible to reuse the autobone because it may provide bacterial culture. Conversely, a 3D printing flap is formed using titanium, which has a lower infection rate. In cases of infection caused by using an autologous skull bone flap, the infection must be overcome through revision surgery using a 3D printed bone flap. In addition, resorption may occur due to apoptosis of the bone flap in the AC18). In such cases, the defect at the cranioplasty site could increase, resulting in a decrease in bone density19).
There was no statistical difference in the operation time and blood loss between the AC and 3DC groups. In all patient groups, operation time and blood loss were required during adhesiolysis of the dura mater from the scalp. In the case of 3D printing flap cranioplasty, multiple holes can be tented with the screw fixation site, and the procedure required during operation has been shortened. In terms of patient satisfaction, all the patients were generally satisfied. In the case of a 3D printing flap, additional manufacturing is possible according to the patient. As it is manufactured based on muscle atrophy and according to the opposite side of the skull, it can be customized to a shape that suits the skull defect. However, in 3D bone flaps, a 1 mm thin-cut CT examination must be additionally performed for preoperative production, and it has the disadvantages of extra cost and radiation exposure risk.
In this study, we evaluated cranioplasty using a 3D printed bone flap and an autobone flap. The 3DC group had a high skull defect coverage rate and a low complication rate.
This was a retrospective medical record study with few cases. However, many artificial materials made by 3D printing techniques are currently used in surgery, and we expect this technique to develop rapidly. Therefore, future studies are essential to 3D-printing medical materials.
CONCLUSION
Cranioplasty using a customized artificial bone flap made by a 3D printing technique was effective for covering skull defects with lower complication rates than the autobone technique.
Supplementary Materials
Further details on supplementary materials are presented online (available at https://doi.org/10.32587/jnic.2021.00388).
Notes
Conflict of interest
No potential conflict of interest relevant to this article was reported.