An Observational Study on the Effect of Nimodipine on Cerebral Blood Flow Velocity and Oxygenation in Patients with Subarachnoid Haemorrhage
Article information
Abstract
Objective
In patients with subarachnoid haemorrhage (SAH), nimodipine is administered to prevent for cerebral vasospasm. In this prospective observational study, we examined the effect of nimodipine on cerebral blood flow velocity (BFV) (transcranial Doppler, TCD) and regional oxygen saturation (rSO2) (near-infrared spectroscopy, NIRS) as well as its systemic haemodynamic effects.
Methods
After ethics approval, we enrolled 18 adult patients (11 females) with SAH after aneurysm rupture. After treatment of the bleeding source, patients underwent extended haemodynamic monitoring by transpulmonary thermodilution (PiCCOTM, Pulsion, Maquet Getinge Group, Feldkirchen, Germany). Measurements of systemic haemodynamics as well as cerebral oxygenation and blood flow velocity were performed 30 minutes before and after the enteral administration of nimodipine (60 mg). Data was compared by paired t-test. A p<0.05 was considered as statistically significant. Data are expressed as mean ± SD.
Results
Patients’ mean age was 59 ± 11 years. Enteral nimodipinincrease in heart rate (75 vs. 79/min, p<0.05) and cardiac index (3.27 vs. 3.58 l/min/m2, p<0.05). While noe administration was associated with a decrease in mean arterial pressure (93 vs. 88 mmHg, p<0.05), an radrenaline dose and BFV did not change significantly, NIRS revealed a reduction in cerebral oxygenation, but rSO2 values remained within a healthy range in all patients (right 71 vs. 69 %, p<0.05; left 74 vs. 72 %, p<0.05).
Conclusions
In patients with SAH, enteral nimodipine led to inconclusive changes in systemic haemodynamics. Although cerebral oxygenation as assessed by NIRS decreased after nimodipine administration, BFV remained stable.
INTRODUCTION
Subarachnoid haemorrhage after aneurysm rupture (SAH) is associated with significant morbidity and mortality6). Delayed cerebral ischemia (DCI) is a clinical syndrome of focal neurological deficits that develop in one third of patients, and is a major cause of death and disability after SAH11,12). Although further aspects like brain injury, inflammation and microthrombosis and their influence on DCI are currently being discussed13), cerebral vasospasms (CVS) remain the most serious complication of SAH, leading to DCI and infarction14). CVS after SAH are described as narrowing of angiographically visible cerebral arteries6) and occur in 70 % of patients with SAH after aneurysm rupture12). Typically, CVS occur between day 3 and 4 after aneurysm rupture, their peak is between day 7 to 10 and they resolve by day 14 to 27,12). Nimodipine remains the only approved medication to prevent DCI9). As a lipophilic antagonist of calcium channels, nimodipine acts as a cerebrovascular vasodilator. In the largest randomized multicenter double-blind placebo-controlled study, nimodipine significantly reduced cerebral infarctions and poor outcome in patients with SAH8). According to the guidelines of the American Heart Association and the American Stroke Association, nimodipine should be administered orally to all patients with SAH (Class I, Level of Evidence A) 6). Therefore, it is routinely administered (60 mg every four hours) from the time of SAH presentation through day 216).
In clinical routine, transcranial Doppler (TCD) is a widely used tool to monitor for the development of SAH-induced CVS by assessing blood flow velocity (BFV) in the intracranial arteries6). Furthermore, near-infrared spectroscopy (NIRS) enables bedside brain monitoring by a non-invasive measurement of regional cerebral oxygen saturation (rSO2) via absorption of near-infrared light of oxyhaemoglobin (HbO), deoxyhaemoglobin (Hb), and cytochrome-oxidase3,13,15,17,18). Since there is still a lack of knowledge, we studied the systemic haemodynamic effects of nimodipine (as assessed by transpulmonary thermodilution with PiCCOTM technology) as well as its impact on BFV and rSO2 (as assessed by TCD and NIRS) in SAH patients.
METHODS
After approval from the institutional review board (Ethics Committee of the University of Witten/ Herdecke, number 48/2012, chair: Prof. Dr. med. P. Gaidzik, date of approval 2 September 2013), written individual informed consent was obtained for patients who presented at our hospital with SAH. In this prospective observational study, we included 18 adult critically ill patients with SAH between August 2014 and June 2018.
After SAH was diagnosed by computed tomography (CT), treatment was achieved by means of open surgical clipping or endovascular aneurysm obliteration. In all patients, an intracranial probe was inserted for continuous measurement of intracranial pressure (ICP). All patients were sedated and mechanically ventilated with continuous measurement of arterial blood pressure, heart rate, oxygen saturation and continuous recording of electrocardiogram. All patients enrolled underwent advanced hemodynamic monitoring with PiCCOTM technology (Pulsion, Maquet Getinge Group, Feldkirchen, Germany) due to poor clinical condition with haemodynamic instability. For the transpulmonary thermodilution measurement, a 15 ml bolus of cold 0.9 % saline was injected via a central venous catheter (V. cava superior). This procedure was repeated three times and the average was used to calculate the thermodilution parameters to calibrate the device for continuous pulse contour analysis. Regional cerebral oxygenation was detected by NIRS (INVOSTM 5100 C, Somanetics, Minneapolis, MN, USA). Self-adhesive oximetry strips were applied bilaterally on the patient’s forehead for continuous measurement of rSO2. TCD was performed to assess BFV of the intracranial arteries (middle cerebral artery (MCA) and anterior cerebral artery (ACA) using device (Multi-Dop®, Singen, Germany).
Nimodipine was routinely administered (60 mg every four hours) via a gastric probe to all patients beginning from the time of SAH presentation. Measurements of systemic and cerebral haemodynamics as well as regional cerebral oxygenation were performed 30 minutes before and after the administration of nimodipine. This time point was chosen since, according to the technical information, nimodipine which after oral administration is detected in blood after 10-15 min with a maximum after about 40 min. Each patient’s examination and data collection were performed by the same examiner.
Data were tested for normal distribution by the Kolmogorov-Smirnov test. Data are expressed as mean ± SD. The effect of nimodipine on systemic and cerebrovascular variables was compared using a paired t-test (Microsoft® Excel). P values less than 0.05 were considered as statistically significant.
RESULTS
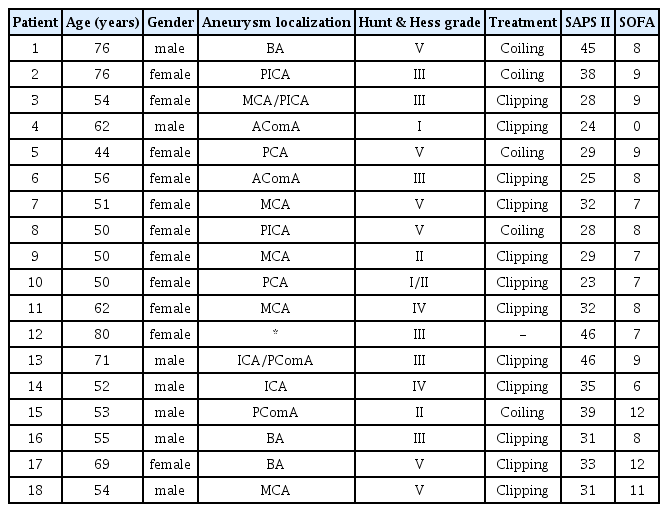
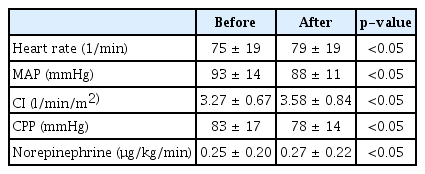
Eighteen patients (mean age 59 ± 11 years, 11 females, 7 males) with SAH were included in this study (Table 1). Twelve aneurysms were treated with clip occlusion and five aneurysms were embolized with coils. In one case no bleeding source was found, hence no treatment was performed in this patient. After nimodipine administration a significant decrease in mean arterial pressure (MAP) (93 vs. 88 mmHg, p<0.05) was observed together with a concomitant increase in heart rate (75 vs. 79/min, p<0.05). Furthermore, cardiac index (CI) increased significantly (3.27 vs. 3.58 l/min/m2, p<0.05) (Table 2). TCD showed normal and stable mean BFV in MCA and ACA before and after nimodipine administration. NIRS revealed a significant bifrontal reduction of rSO2 (right 71 vs. 69 %, p<0.05; left 74 vs. 72 %, p<0.05), yet staying within a physiological range among the study population (Table 3).
Systemic haemodynamics and vasopressor support before and 30 minutes after enteral administration of 60 mg of nimodipine
Variables of cerebral blood flow velocity and oxygenation before and 30 minutes after enteral administration of 60 mg of nimodipine
Elevated BFV of both MCA and ACA was seen in one patient, with values up to 200 cm/sec (Fig. 1A and B). In this patient, nimodipine administration was associated with a further bilateral increase of BFV of MCA and ACA. While rSO2 values remained without significant change after nimodipine administration, NIRS detected a non-significantly lower right sided cerebral oxygen saturation (69 vs. 81 %) consistent with a considerably high BFV in MCA on the same side.
DISCUSSION
In patients with SAH, we found that enteral nimodipine administration was associated with hypotension, an increase in heart rate and slightly higher noradrenaline requirements, while CI increased significantly. No significant changes in BFV in MCA and ACA were detected by TCD. Interestingly, NIRS revealed a decrease in frontal regional cerebral oxygenation, probably due to impaired cerebral autoregulation, however rSO2 values were within a physiological range in all patients.
In healthy subjects, nimodipine has shown to be effective in increasing cerebral perfusion in spite of its hypotensive effect. Similar to our results, a decrease in arterial blood pressure with a concomitant increase in heart rate appeared without significant change of mean BFV in MCA. However, in contrast to our data, NIRS-derived regional oxygenation saturation improved after nimodipine administration4).
In patients with SAH, the effect of nimodipine is ambiguous. Nimodipine seems to be unable to alter the incidence or severity of vasospasm6,12), yet it reduces the risk of DCI and improves neurologic outcome after SAH12). If clinically significant DCI occurs, arterial hypertension is recommended to improve cerebral perfusion12). While several studies report increased cerebral blood flow (CBF) due to nimodipine, few show reduced CBF linked to decreased MAP and CPP5,10,16). In contrast, one retrospective study even revealed an enhanced risk of CVS with a higher need for vasopressor agents associated with nimodipine administration10). Nimodipine is routinely used to prevent CVS, but its effect on patients with existing CVS might be risky. When nimodipine induces hypotension, an impaired autoregulation might lead to reduced cerebral perfusion with decreased rSO2 values in these patients. It remains unclear how many patients were indeed affected by CVS in the present study. TCD is widely used to consider CVS if mean BFV in MCA exceeds 120 cm/sec10). Overall, mean BFV in our patients remained within a physiological range. A pathologic increase of BFV only occurred in one patient in whom we found even further acceleration of BFV after nimodipine administration. In this case, rSO2 showed normal and stable values.
So far, no clear recommendation regarding the interpretation of NIRS values exists. Maslehaty et al. suggests rSO2 below 40 % or a decrease by 25 % from the baseline to be associated with DCI and neurological deficits13). This did not appear in any of our patients. Either nimodipine administration did not lead to an improvement of cerebral perfusion and oxygenation as mean BFV and rSO2 remained within the normal range anyway, or the used methods were inadequate to detect a possible improvement.
When interpreting TCD- and NIRS-derived values, one should consider that TCD mainly assesses the MCA area, while bilaterally applied oximetry sensors on the forehead mainly involve the ACA territory and only partially covers the important MCA area13). A modified application of the sensors should be considered. However, regardless of the applied location, near-infrared light only penetrates 2-2,5 cm into the head, hence NIRS only reflects the cortical region without assessing deeper regions of the brain14). In addition, NIRS measurements can be tampered by factors like fever or sweat of the skin13).
Though NIRS succeeded to detect improvement of rSO2 after intraarterial infusion of fasudil hydrochloride, a potent vasodilator, to a patient suffering from SAH-induced CVS14), its diagnostic value needs to be further investigated. Whether other methods as digital substraction angiography or perfusion CT would be able to detect an impact of nimodipine on BFV remains uncertain, too.
Our study has several limitations. As a lipophilic vasodilator, we expected nimodipine to cause cerebrovascular dilation with elevated CBF and rSO2. Due to its low bioavailability and in accordance with previous studies1,2,10), it is conceivable that the gastric administration of nimodipine failed to attain therapeutic plasma concentrations needed to achieve the desired effect. However, in this clinical study, nimodipine plasma concentrations were not measured and hence a dose-effect relation cannot be analyzed. Furthermore, the sample size and the heterogeneity of the patient population are major limitations of this observational study.
CONCLUSIONS
In summary, enteral nimodipine is routinely used to prevent DCI, which can occur due to CVS in patients with SAH. In our study population, nimodipine showed inconclusive changes in systemic haemodynamics. BFV in MCA and ACA remained stable, not supporting the idea of cerebrovascular dilation following nimodipine administration. It remains unclear why rSO2 decreased bilaterally, when we assume the absence of CVS due to overall normal BFV in this study population. Further studies are required to conclude the cerebral effect of enteral nimodipine in patients with SAH.
Notes
Conflict of interest
No potential conflict of interest relevant to this article was reported.