Predictors for Unfavorable Outcomes and Recurrence after Endovascular Treatment for Ruptured Intracranial Aneurysms
Article information
Abstract
Objective
With current increase in the use endovascular treatment (EVT) for ruptured aneurysms, it is important to recognize factors associated with unfavorable outcomes and recurrence in patients with subarachnoid hemorrhage (SAH) treated with endovascular coiling. This study aimed to identify the predictors of unfavorable outcomes and recurrence in patients who received EVT for ruptured aneurysms.
Methods
102 patients who were treated with an endovascular procedure for a ruptured aneurysm, including both stent-assisted and non-stent-assisted coil embolizations, were enrolled in this study. A retrospective analysis was performed to identify significant predictors of unfavorable outcomes and recurrence.
Results
At the last follow-up, 72 patients (70.59%) showed a favorable outcome (modified Rankin Scale [mRS] score: 0–2), while 30 patients (29.41%) showed an unfavorable outcome (mRS score: 3–6). In the univariate and multivariate logistic regression analyses, the following variables were found to be the significant predictors of unfavorable outcomes among patients with all-grade SAH: initial Hunt–Hess grade (p = 0.02), periprocedural complications (p = 0.01), and external ventricular drainage (EVD) (p = 0.03), while those among patients with good-grade SAH (Hunt–Hess grades 1–3) were as follows: age (p = 0.009), re-bleeding before treatment (p = 0.002), EVD (p = 0.003), and delayed cerebral ischemia (p = 0.04). Further, the aneurysm volume (p = 0.043) and initial Raymond classification (p = 0.04) were found to be significantly correlated with recurrence.Conclusion: There are various predictors of unexpected unfavorable outcomes and recurrence in patients who receive EVT for ruptured aneurysms. Therefore, careful individualized consideration is necessary for patients with acute SAH who plan to receive EVT.
INTRODUCTION
Endovascular treatments for ruptured aneurysms have progressively replaced clipping since the International Subarachnoid Aneurysm Trial was published in 20026,13,19,21,29). In addition, the rapid advancements in endovascular technology have expanded the indications for securing ruptured aneurysms using endovascular treatment1). However, subarachnoid hemorrhage (SAH) remains associated with an overall mortality of approximately 50%2). Even in survivors of SAH, unfavorable neuropsychological outcomes are common, and the recurrence rate is relatively high15,23). Previous studies have identified several factors associated with unfavorable outcomes or recurrence in SAH16,25,26). However, most of these studies were performed on heterogeneous patient groups that received surgical clipping or endovascular coiling. To the best of our knowledge, risk factor analyses of unfavorable outcomes and recurrence in patients with aneurysmal SAH treated with endovascular coiling only are scarce30). With the current increase in the use of endovascular treatment for ruptured aneurysms, it is important to recognize factors associated with unfavorable outcomes and recurrence in patients with SAH treated with endovascular coiling deserves emphasis. Therefore, we performed a retrospective analysis to identify the predictors of unfavorable outcomes and recurrence in patients with SAH who received endovascular treatment.
MATERIALS AND METHODS
General
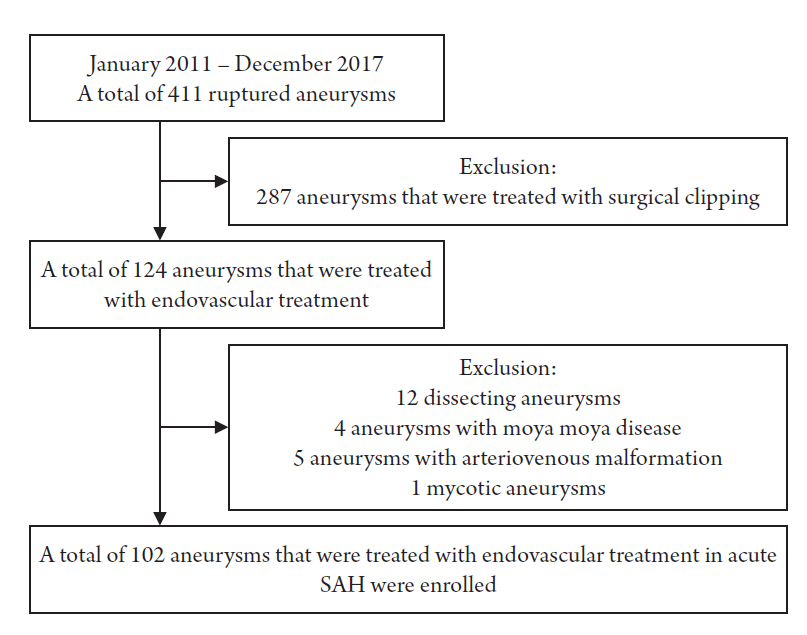
A total of 411 ruptured aneurysms were treated in our institute between January 2011 and December 2017, and 124 of these were treated with an endovascular method (287 aneurysms treated with surgical clipping were excluded). Of these 124 aneurysms, 12 dissecting aneurysms treated with stents only, 4 aneurysms related to Moyamoya disease, 5 aneurysms with arteriovenous malformation, and 1 mycotic aneurysm were excluded. Therefore, a total of 102 aneurysms that were treated with an endovascular method, including both non-stent-assisted coil embolization and stent-assisted coil embolization (SAC), in 102 patients were included in the study (Fig. 1).
Clinical outcomes were measured using the modified Rankin Scale (mRS) at the last follow-up. Based on the mRS score at the last follow-up, patients were categorized into the following two groups: favorable outcome (mRS score: 0–2) and unfavorable outcome (mRS score: 3–6). Before analyzing the clinical aggravation factors in patients with good-grade SAH, we defined good-grade SAH as SAH with an initial Hun–Hess scale score of 1–3 and poor-grade SAH as SAH with an initial Hunt–Hess scale score of 4–5. The volumes of the aneurysms were measured using a website-based open source calculator (http://www.angiocalc.com; Angiocalc® LLC, Charlottesville, Virginia) and were based on their digital subtraction angiographic findings. Any aneurysm with an increased filling of contrast in the neck or body was considered a recurrence, regardless of the need for retreatment. All procedure-related complications were also reported, regardless of their clinical significance. Thromboembolic complications were confirmed as intraprocedural angiographic evidence or brain magnetic resonance imaging findings that were consistent with those for acute cerebral infarction. Hydrocephalus was diagnosed using brain computed tomography (CT) in the presence of neurological deterioration. Delayed cerebral ischemia (DCI) was diagnosed as the presence of vasospasm after careful exclusion of other causes.
Our institutional review board approved this retrospective study, and the need for informed consent was waived owing to its retrospective nature.
Endovascular treatment
All procedures were performed under general anesthesia. Systemic heparinization was maintained in all patients via the administration of normal saline mixed with heparin at a concentration of 3 units of heparin per 1 mL of saline. After the achievement of sufficient aneurysm obliteration, an intravenous 50 U/kg dose of heparin was injected as a bolus, and for achieving a 2.5-fold higher level of the baseline activated clotting time during the procedure, several intermittent boluses of heparin were additionally administered. In patients treated with SAC, immediate postoperative administration of 100 mg acetylsalicylic acid and 400 mg clopidogrel was performed; thereafter, 100 mg acetylsalicylic acid and 75 mg clopidogrel were administered daily for at least 3 months followed by life-long administration of 100 mg acetylsalicylic acid. The decision to perform SAC was based on the aneurysm morphology and the relationship between the parent artery and aneurysm. Self-expanding stents (mainly Enterprise®; Cordis, Miami Lakes, Florida) were used in all cases of SAC for wide-necked aneurysms (>4 mm) and for aneurysms with an unfavorable dome-to-neck ratio (<1.5) and as a rescue method when the coils bailed out into the parent artery. The aneurysms were embolized with various bare-platinum coils at the surgeon’s preference. Radiographic follow-up using digital subtraction angiography (DSA), computed tomography angiography (CTA), or magnetic resonance angiography (MRA) was conducted after endovascular treatment as part of a routine protocol at our hospital; plain skull radiography was conducted at 3, 6, and 12 months after treatment, MRA at 6 and 24 months after treatment, and DSA at 1 and 3 years after treatment if there were abnormal findings on CTA or MRA.
Statistical analysis
Data were collected and expressed as means and standard deviations for continuous variables and frequencies or percentages for categorical variables. We analyzed these variables using an unpaired t-test, the chi-square test, and Fisher’s exact test. Multivariate and univariate logistic regression analyses for unfavorable outcomes and recurrence were conducted. For the multivariate logistic regression analysis, all factors that showed significance in the univariate logistic regression analysis were included. A prognostic model that included the significant factors in the multivariate logistic regression analysis for unfavorable outcomes was created, and the area under the receiver operating characteristic curve (AUROC) was used to test the model’s prediction ability. An AUROC of >0.80 indicated excellent discrimination.15 A p-value of ≤0.05 was considered statistically significant. All analyses in the present study were performed using the Statistical Package for Social Sciences (Version 21; IBM, Armonk, New York).
RESULTS
Favorable outcomes versus unfavorable outcomes
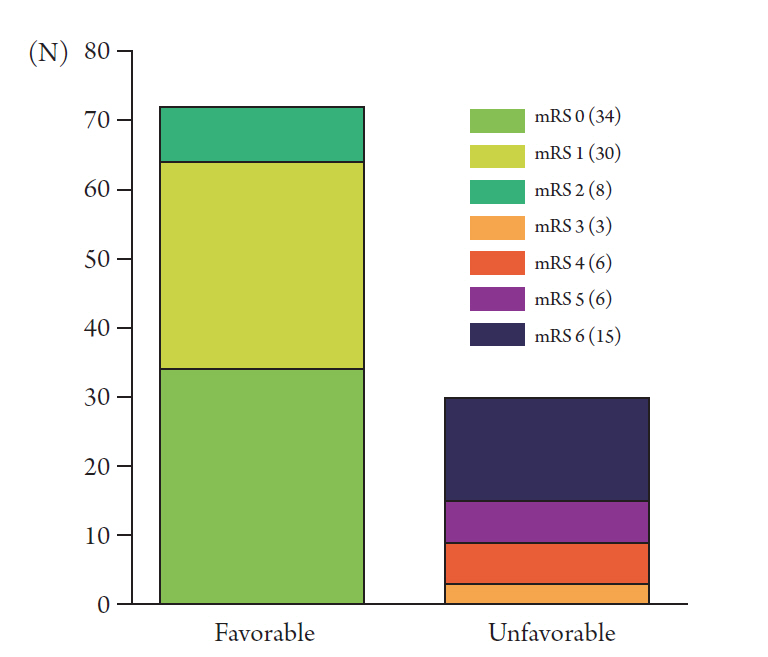
The baseline demographics and details of favorable outcome and unfavorable outcome groups are shown in Table 1. The mean clinical follow-up duration of the entire cohort was 790.00 (range, 8–1869) days. A total of 71 patients were available for angiographic follow-up, and their mean angiographic follow-up duration was 704.69 (range, 176–1855) days. At the last follow-up, 72 patients (70.59%) showed a favorable outcome, while 30 patients (29.41%) showed an unfavorable outcome (Fig. 2). Of the 15 patients who showed an mRS score of 6 (dead) at the last follow-up, 10 patients died from increased intracranial pressure during hospitalization. Therefore, the rate of in-hospital mortality was 9.80%.
The comparison analysis between the two groups showed that patients in the unfavorable outcome group were older than those in the favorable outcome group (p < 0.001). In addition, there were significant differences in the initial Glasgow coma scale (GCS) score, Hunt–Hess grade, and modified Fisher grade between the two groups. The unfavorable outcome group constituted more patients treated with external ventricular drainage (EVD) in the acute phase of SAH than the favorable outcome group (60.00% vs. 12.50%, p < 0.001, odds ratio [OR] = 10.500, 95% confidence interval [CI] = 3.82–28.84). Of the entire cohort, a total of six patients underwent decompressive craniectomy after their ruptured aneurysms were secured to reduce their increased intracranial pressure. The unfavorable outcome group constituted more patients who underwent decompressive craniectomy than the favorable outcome group (p = 0.008, OR = 14.200, 95% CI = 1.581–127.501). A total of six patients had re-rupture of their ruptured aneurysm before treatment; among these patients, five showed an unfavorable outcome and one was discharged with mild memory impairment and a headache.
No significant differences in the aneurysm characteristics were observed between the two groups. The overall rate of periprocedural complications was 13.72%, including nine cases of intraprocedural thromboembolism (8.82%), one case of re-rupture during the procedure (0.98%), three cases of remote hemorrhage (2.94%), and one case of re-bleeding after successful embolization (0.98%). The rate of periprocedural complications in the unfavorable outcome group was higher than that in the favorable outcome group (33.33% vs. 5.56%, p = 0.001, OR = 8.50, 95% CI = 2.40–30.03). Compared to patients in the favorable outcome group, a larger number of patients in the unfavorable outcome group needed shunt operations for hydrocephalus (33.33% vs. 15.28%, p = 0.04, OR = 2.77, 95% CI = 1.02–7.49). The rate of DCI in the two groups was similar. The rate of recurrence in all patients was 29.58%, and there was no significant difference between the two groups.
Predictors of unfavorable outcomes
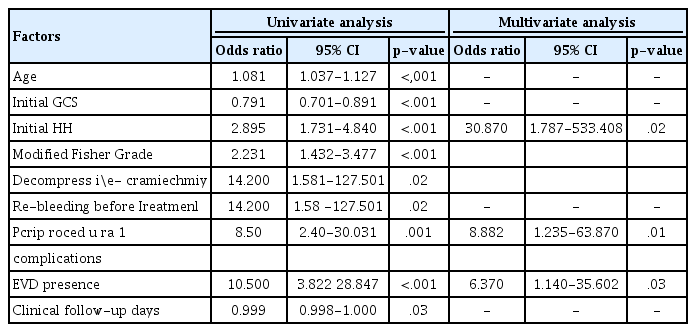
The results of the univariate and multivariate logistic regression analyses for unfavorable outcomes are presented in Table 2. In the univariate logistic regression analysis, older age (p < 0.001), initial lower GCS score (p < 0.001), initial higher Hunt–Hess grade (p < 0.001), higher modified Fisher grade (p < 0.001), decompressive craniectomy (p = 0.02), re-bleeding before treatment (p = 0.02), periprocedural complications (p = 0.001), EVD (p < 0.001), and longer clinical follow-up duration (p = 0.03) were significantly associated with unfavorable outcomes. Aneurysm geometry, such as the size of the dome or neck, and volume were not relevant factors. In the multivariate logistic regression analysis, a higher initial Hunt–Hess grade (p = 0.02), periprocedural complications (p = 0.01), and EVD (p = 0.03) were found to be the significant predictors associated with unfavorable outcomes.
Subgroup analysis for the unfavorable outcomes of patients with good-grade SAH
Our cohort included 75 patients with good-grade SAH (initial Hunt–Hess grades 1–3) and 27 patients with poor-grade SAH (initial Hunt–Hess grades 4–5).
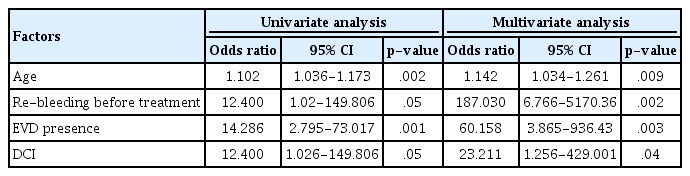
The results of the univariate and multivariate logistic regression analyses for the predictive factors of unfavorable outcomes in patients with good-grade SAH are presented in Table 3. In the univariate logistic regression analysis, older age (p = 0.002), re-bleeding before treatment (p = 0.05), EVD (p = 0.001), and development of DCI (p = 0.05) were significantly associated with unfavorable outcomes; similarly, all these factors were identified as the significant predictors of unfavorable outcomes in the multivariate logistic regression analysis. However, the analysis of patients with poor-grade SAH did not reveal any significant predictive factors of unfavorable outcomes (results not shown in the table).
Prognostic model for unfavorable outcomes
Prognostic models that were based on the findings of the multivariate logistic regression analysis of unfavorable outcomes were created for patients with all-grade SAH and good-grade SAH (Fig. 3). The prognostic model for patients with all-grade SAH included the initial Hunt–Hess grade, EVD, and periprocedural complications. The prognostic model for patients with good-grade SAH included age, re-bleeding before treatment, DCI, and EVD. Both prognostic models showed excellent discrimination; the prognostic model for unfavorable outcomes in patients with all-grade SAH had an AUROC of 0.877 (p < 0.001, 95% CI = 0.807–0.947) and that in patients with good-grade SAH had an AUROC of 0.909 (p < 0.001, 95% CI = 0.784–1.000).

Area under the receiver operating characteristic curves (AUROCs) for the prediction of undesirable outcomes in the prognostic models. (Left) The prognostic model of unfavorable outcomes for the patients with all-grade subarachnoid hemorrhage (SAH) had an AUROC of 0.877 (p < 0.001, 95% confidence interval [CI] = 0.807–0.947). (Right) The prognostic model of unfavorable outcomes for the patients with good-grade SAH had an AUROC of 0.909 (p < 0.001, 95% CI = 0.784–1.000).
Predictors of recurrence
The following factors were found to be the significant predictors of recurrence in the univariate logistic regression analysis: larger aneurysm neck diameter (p = 0.02), larger maximal aneurysm diameter (p = 0.02), greater aneurysm height (p = 0.01), larger aneurysm volume (p = 0.04), and initial Raymond classification (p = 0.04). Conversely, the following factors were found to be the significant predictors in the multivariate logistic regression analysis: aneurysm volume (p = 0.04) and initial Raymond classification (p = 0.04) (Table 4).
Subgroup analysis for recurrence (SAC versus coil embolization without stenting)
The following factors were tested in the univariate logistic regression analysis of patients treated with coil embolization without stenting: age (p = 0.43), aneurysm location (p = 0.25), aneurysm neck (p = 0.009), aneurysm diameter (p = 0.03), aneurysm height (p = 0.06), aneurysm volume (p = 0.06), initial Raymond classification (p = 0.007), and angiographic follow-up duration (p = 0.51). However, only the following factors were found to be significantly correlated with recurrence in the multivariate logistic regression analysis: aneurysm neck (p = 0.04) and initial Raymond classification (p = 0.03) (Table 5). These same factors were tested in the univariate logistic regression analysis of patients treated with SAC. However, no significant predictors of recurrence of aneurysm in patients treated with SAC were observed: age (p = 0.62), aneurysm location (p = 0.93), aneurysm neck (p = 0.25), aneurysm diameter (p = 0.24), aneurysm height (p = 0.10), aneurysm volume (p = 0.36), initial Raymond classification (p = 0.98), and angiographic follow-up duration (p = 0.60).
DISCUSSION
The current study demonstrated that various clinical variables are associated with unfavorable outcomes or recurrence in patients with SAH who are treated with endovascular coil embolization. The prognostic models that included the significant factors in the multivariate logistic regression analysis revealed an excellent discrimination of unfavorable outcomes in patients with all-grade SAH and good-grade SAH.
Aneurysmal SAH has a mortality rate of 50% and is a leading cause of cerebrovascular mortality5,14,18,20,27). Since the introduction of endovascular methods for treating cerebral aneurysms by Guglielmi et al., these treatment strategies are currently accepted, with surgical clipping of the neck of the aneurysm as the standard treatment modality for cerebral aneurysms1,4,6,7,11,21,29). Traditionally, the predictive factors of unfavorable surgical outcomes in patients with aneurysmal SAH included the following: poor initial neurological status, presence of hydrocephalus, large aneurysm, complexity of the anatomy, high Fisher grade, presence of a posterior circulation aneurysm, and old age25). However, the predictors for patients who receive endovascular treatment only for ruptured aneurysms have not been extensively studied30).
Our comparison analysis between the favorable and unfavorable outcome groups revealed results almost identical to those of previous studies that also included patients treated with surgical clipping16,25,26). Old age, poor initial neurological status, high Fisher grade, EVD, decompressive craniectomy for poor-grade SAH, and re-bleeding before treatment were the significantly different factors observed between the two groups. However, the geometry of the aneurysm was not a relevant factor. Our results can be explained by the advent of endovascular treatment with fewer technical limitations related to brain swelling and no brain manipulation. Geometrical complexity can be overcome by various dedicated techniques of endovascular treatment for ruptured aneurysms, such as multiple catheterization, balloon assistance, and stent assistance. These techniques can increase the rate of a thromboembolic complication; however, such complications can be prevented by adequate heparinization and postoperative medication. Several studies have shown that there is no significant difference in the clinical outcomes between stent-assisted and non-stent-assisted techniques for ruptured aneurysms3,29).
The multivariate logistic regression analysis in our study revealed that the initial Hunt–Hess grade, periprocedural complications, and need for EVD were predictive factors of unfavorable outcomes. However, neurologically poor patients frequently require EVD for intracranial pressure relief. Therefore, the initial Hunt–Hess grade and periprocedural complications are the truly meaningful predictive factors of unfavorable clinical outcomes. In our cohort, the periprocedural complication rate was high at 13.72%. The majority of complications were thromboembolic in nature, which should be addressed with a delicate medical prevention protocol12).
During postoperative care, we frequently encountered clinical aggravation in patients with an initial good-grade SAH. Owing to the good preoperative condition of patients with good-grade SAH, clinical aggravation was easy to identify in this subpopulation of patients. Therefore, a subgroup analysis of our cohort was performed to compare patients with good-grade SAH and poor-grade SAH and identify the cause of aggravation. The subgroup analysis revealed that old age, re-bleeding before treatment, EVD, and DCI were significantly associated with unfavorable outcomes among patients with an initial good-grade SAH. Numerous previous studies have reported that re-bleeding before treatment is highly associated with unfavorable outcomes and a high mortality rate17,22,25). In addition, DCI is also a well-known severe complication in patients with SAH and occurs in 19%–63% of patients with acute SAH8,10). Our subgroup analysis results for patients with good-grade SAH suggest that early obliteration of the ruptured aneurysm and aggressive monitoring, prevention, and management of DCI are mandatory for the treatment of these patients. Because old age and the need for EVD are fixed factors that cannot be altered, other modifiable factors, such as early treatment of ruptured aneurysms and management with tight surveillance of DCI, should be addressed with high attention.
We found no significant predictors of unfavorable outcomes in patients with poor-grade SAH. However, many previous studies have suggested significant factors that are related to clinical outcomes in patients with poor-grade SAH5,16,26,27). Shirao et al. reported that older age, a high Fisher grade, and a low density area associated with vasospasm on CT were independent predictors of unfavorable clinical outcomes in patients with poor-grade SAH (World Federation of Neurosurgical Society grades 4–5) treated with surgical clipping or endovascular coiling26). Schuss et al. reported that various predictors were significantly associated with unfavorable outcomes in patients with poor-grade SAH; however, they indicated that an early and aggressive treatment could result in favorable outcomes in up to 24% of these patients25).
Recurrence is the primary disadvantage of endovascular coiling compared to clipping, and many studies have been published on recurrence and its prevention9,23). The rates of recurrence vary from 17% to 90%23).The rate of recurrence in this study was 29.58%, and the significant predictive factors of recurrence in our cohort were the volume of the aneurysm and initial Raymond classification. Numerous previous studies have reported a lower risk of recurrence with SAC than with coil embolization without stenting24). However, the rate of recurrence in patients treated with SAC in our study was similar to that in patients treated with coil embolization without stenting. The underlying mechanism for this similarity is not clear, but it may be related to the follow-up loss and the uneven follow-up durations in our cohort.
The subgroup analysis of the predictive factors of recurrence in coil embolization without stenting showed the aneurysm neck size and initial Raymond classification as the predictive factors. However, no predictive factors were found in patients treated with SAC. Thus, we hypothesized that the aneurysm neck covered by a deployed stent could make its size insignificant to induce recurrence.
There are several limitations in our study. This study was a retrospective, single-center study with a relatively small sample size. Radiologic follow-ups were not performed for all patients. Almost-ruptured aneurysms located in the middle cerebral artery were treated with surgical clipping rather than endovascular treatment at our institute; therefore, there was patient selection bias, and the effect of the location of the aneurysm was not considered in the analysis. The physiological condition and combined morbidity of individualized patients, such as pneumonia or cardiac disease, were also not considered in this study.
CONCLUSIONS
In this study, a high initial Hunt–Hess grade, periprocedural complications, and need for EVD were the predictive factors of unfavorable outcomes in patients with SAH treated with endovascular coil embolization. The factors associated with clinical aggravation in patients with good-grade SAH were age, re-bleeding before treatment, and development of DCI. Therefore, every patient with good-grade SAH should be treated with early intervention and thorough strategies to reduce periprocedural complications and manage DCI. Recurrence was related to the aneurysm volume, neck size, and initial obliteration rate; this implicates that the pursuit of complete embolization is required for reduction of the recurrence rate.
Notes
Conflict of interest
No potential conflict of interest relevant to this article was reported.