Hyaluronidase Injection to Reduce Periorbital Edema after Surgery for Unruptured Intracranial Aneurysms
Article information
Abstract
Background
In the era of endovascular treatment for intracranial aneurysms, microsurgical treatment might necessitate the minimization of postoperative discomforts such as headache, nausea, vomiting, and edema. The current study aimed to evaluate the effectiveness of hyaluronidase on the reduction of periorbital edema after clipping surgery for unruptured intracranial aneurysms.
Methods
From July 2016 to March 2018, the patients who underwent elective surgery for unruptured intracranial aneurysms were included. A mixture of 4500 IU hyaluronidase was injected subcutaneously on the sectional plane of the scalp incision. The photographs of patients’ faces were obtained on postoperative day 1, 3, and 7. The degree of periorbital edema was judged on the grading system based on the vertical palpebral aperture. The thickness of the scalp, temporalis muscle, and soft tissue was measured on a preoperative computed tomography (CT) scan, and other variables (e.g., intraoperative input and output, serum albumin level) were analyzed retrospectively.
Results
A total of 60 patients were included in this study, and a hyaluronidase mixture was injected in 30 patients. The periorbital edema tended to resolve in 7 days after surgery, but notable differences between the two groups was observed at day three after surgery in this study. On the ordinal logistic regression analysis on the grade of periorbital edema, hyaluronidase injection is significantly associated with the reduction of periorbital edema throughout the postoperative period. (p value < 0.05)
Conclusion
Hyaluronidase injection might be helpful in reducing periorbital edema after surgery for unruptured intracranial aneurysms.
INTRODUCTION
In Korea, the number of endovascular treatments for intracranial aneurysms has overwhelmed the number of microsurgical clipping since 2014, based on the data of the Health Insurance Review & Assessment Service (HIRA)1). This trend might result from the superiority in accessibility and recoverableness of the endovascular treatment over microsurgical clipping. Accordingly, several studies have been conducted to reduce postoperative headache, nausea, and vomiting, and to reduce postoperative edema after craniotomy2-4). Furthermore, these efforts might be the elements of Enhanced Recovery After Surgery (ERAS) strategies for craniotomy, which has been adopted more recently compared with other specialties5).
Especially, periorbital edema after craniotomy is reported frequently during the postoperative period up to 79.5% and it might be accompanied by pain and ecchymosis6). Cryotherapy was reported as effective in reducing postoperative edema, but it might be applied in contact with skin postoperatively3). Hyaluronidase is known as a spreading factor and is reported to reduce periorbital edema after plastic procedures7). However, there has not been any study using hyaluronidase for periorbital edema in craniotomy. We expected the hyaluronidase might reduce periorbital edema after craniotomy based on the properties of which would promote the diffusion and dispersion of fluids and wound healing8). The current study aimed to evaluate the effectiveness of hyaluronidase on the reduction of periorbital edema after clipping surgery for unruptured intracranial aneurysms.
MATERIALS AND METHODS
Study design
After the authors had received approval from the Institutional Review Board, the retrospective analysis was performed on a total of 280 patients who were treated for unruptured aneurysms surgically between June 2016 and March 2018. Among 280 patients, 60 patients were included in the current study based on the following inclusion criteria.: 1) scheduled microsurgical clipping of unruptured aneurysms, located on anterior circulation 2) pterional approach or lateral supraorbital approach 3) available photograph of patient’s face including both peri-orbital region on postoperative day 1, 3, and 7.
In addition to demographic data of included patients, the thickness of scalp and muscle was measured on preoperative computed tomography (CT) or magnetic resonance images (MRI), and intraoperative volume input and output (I/O), retraction time, pre-operative and post-operative serum albumin was collected, based on medical record. Moreover, it was identified whether the patient had surgical drains and whether the patient took streptokinase/streptodornase (Varidase TM, SK Chemicals, Korea) on postoperative day 1 or 2. Moreover, to identify the relation between periorbital edema and subjective pain, the first pain score immediately after the surgery and the maximum pain score of postoperative day 0, 1, 3, and 7 was collected using a numeric pain intensity scale (NPIS) 9).
Surgical procedures
Microsurgical clipping of unruptured aneurysm was undergone as a general principle. Before scalp closure, a mixture of hyaluronidase 3000 I.U. (H-lase inj, Kuhnil Pharmaceuticals, Korea), 1 milliliter (ml) of 1:10000 lidocaine/epinephrine and 10ml of normal saline was injected into the subcutaneous layer of the scalp flap along the incision line. During the procedure, retraction time was defined as the interval between making burr holes and re-approximating the bone flap, which was defined as operation time on the anesthesiologist’s record. We usually utilized drainage catheters for the patients with conventional pterional approach otherwise we did not use them for the patients with mini-pterional approach or supraorbital approach.
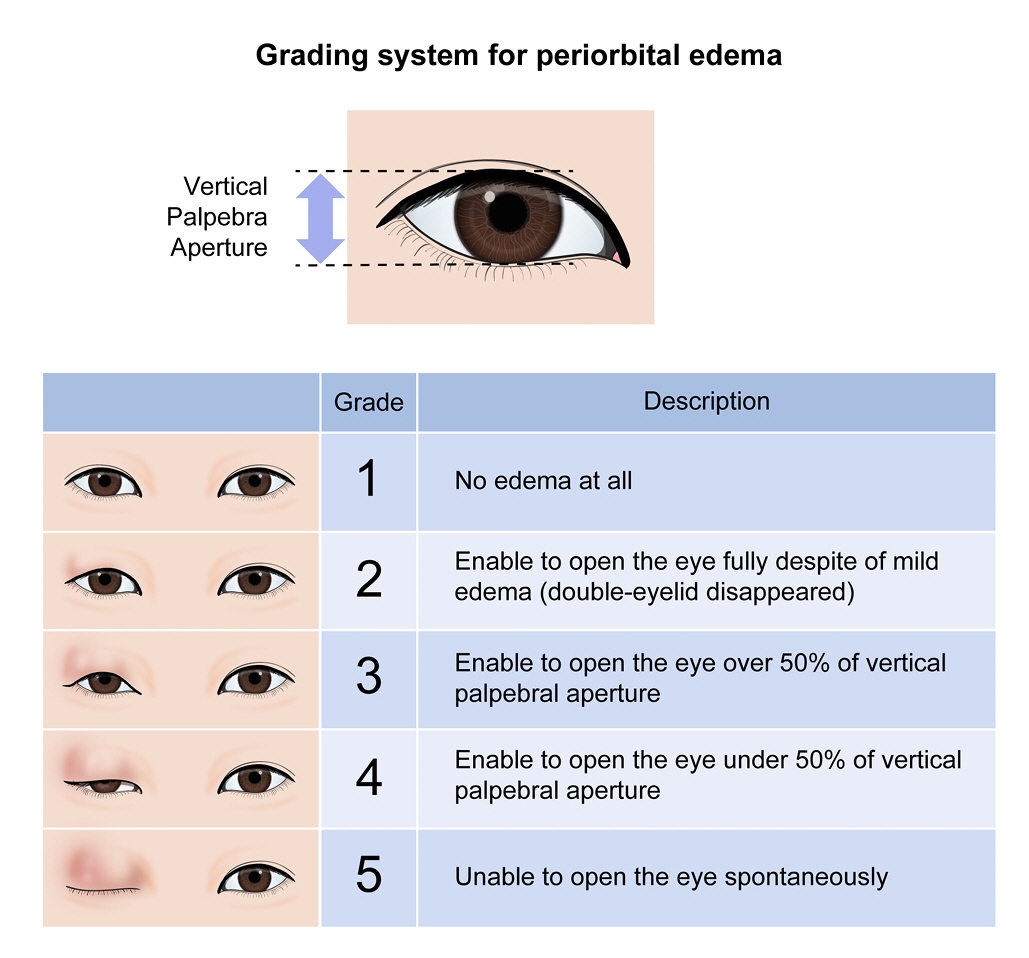
Grading system of periorbital edema
Photographs of patients’ faces were taken before wound dressing on postoperative day 1, 3, and 7. We made a grading system of periorbital edema based on the vertical palpebral aperture of the contralateral eye (Fig. 1). The grading system ranged from 1 to 5 and the higher score means the more severe periorbital edema. Photographs were cropped and the bilateral periorbital region remained. Two physicians independently reported the grade of periorbital edema for the cropped photographs based on the grading system. In case of discrepancy of grades between two physicians (SY and CKJ), the final grading was decided with agreement among the authors (SY, CKJ, JJK).
Radiologic evaluation for muscle thickness
The thickness of the scalp was measured on the axial image of preoperative CT or MRI, at the level of just above ipsilateral orbital roof, which was thought mostly hinged during the surgery. Virtual line for measurement was made perpendicular to the long axis of temporalis muscle and it passed the tip of sphenoid ridge on axial image10). The measurement was performed in detail to evaluate the effect of muscle and soft tissue separately, such as bone-to-deep fascia, bone-to-superficial fascia, and soft tissue.
Statistical analysis
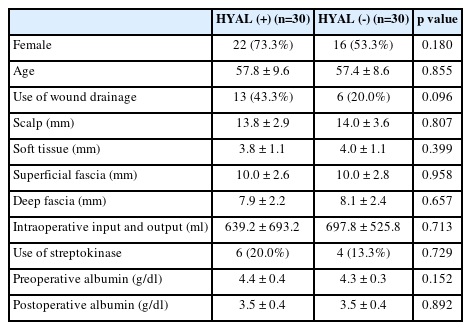
We dichotomized all included patients based on the use of hyaluronidase (HYAL), which are HYAL (+) group and HYAL (-) group. All demographic data was compared using chi-square test or Fischer’s exact test for categorical variables and independent two-sample t-test for continuous variables. To evaluate efficacy of hyaluronidase and identification of predictors for reduction of periorbital edema, we compared ordinal grades of periorbital edema using the ordinal logistic regression model. Ultimately multivariate ordinal logistic regression analysis was performed for significant variables of univariate analysis (p-value < 0.05). We utilized linear mixed model – random intercept model to quantify the relationship of periorbital edema or subjective pain with use of hyaluronidase over time. P value < 0.05 was considered statistically significant in the analyses. SAS software version 9.3 (SAS Institute Inc., Cary, NC, USA) was used for statistical analyses.
RESULTS
Patient demographics
A total of 60 patients were included in this study. There was no significant between HYAL (+) group and HYAL (-) group in baseline demographic and clinical characteristics (Table 1). 61.7% were female patients, and the median age was 57.1 ± 9.4 years. The mean volume of intraoperative I/O was larger in HYAL (+) group but was not significant.
Periorbital edema
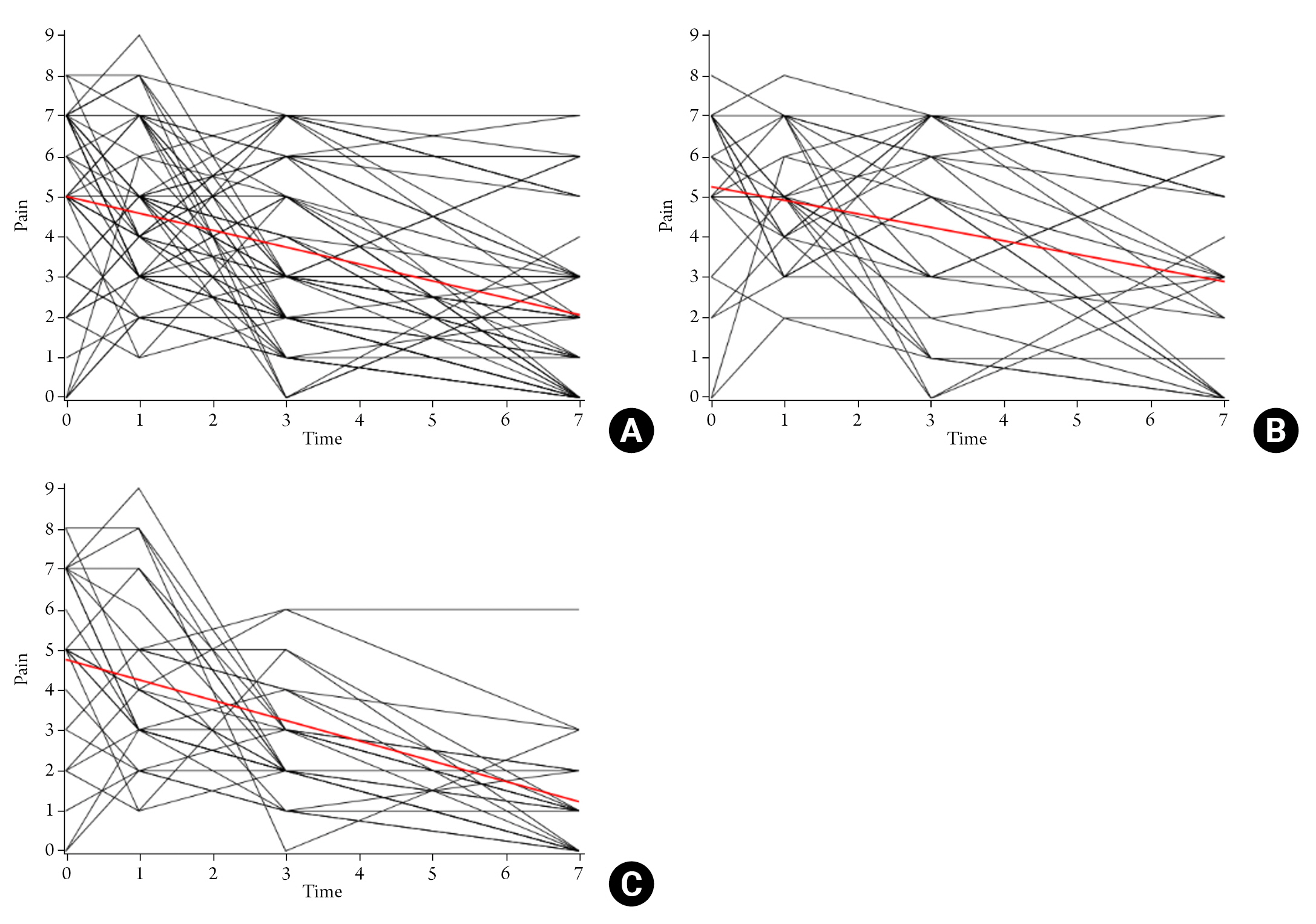
The periorbital edema tended to be increased between postoperative day 1 and 3, and then decreased after postoperative day 3. On the spaghetti plot, the decline of the periorbital edema was identified as steeper in HYAL(-) group than in HYAL(+) group (Fig. 2). However, the decline in both groups was not significantly different with the linear mixed model – random intercept model, which compares the estimated slope of both groups along the time (p=0.287).
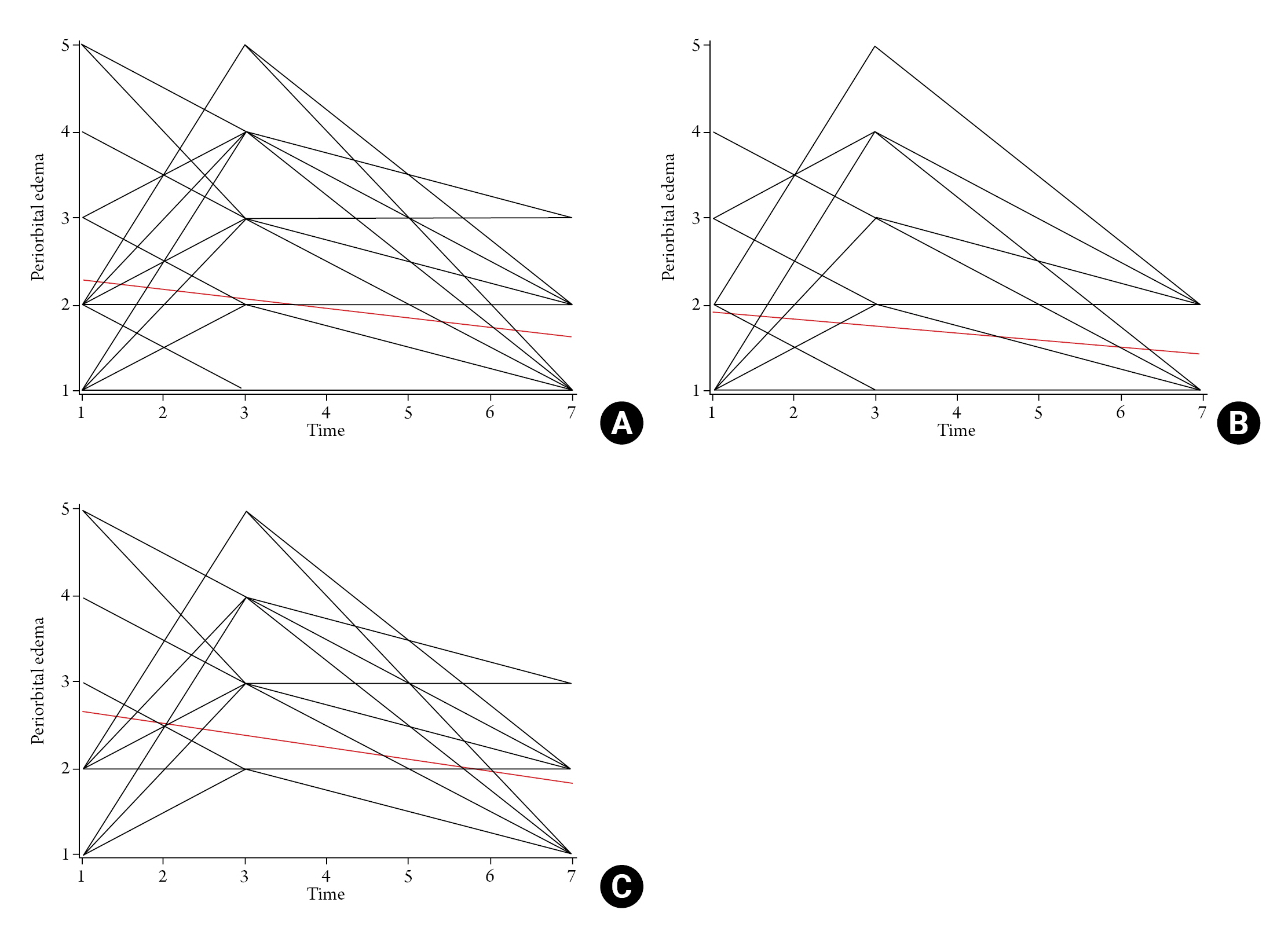
Spaghetti Plot of Periorbital Edema and Postoperative Day. (A) Total population, (B) HYAL(-) group, (C) HYAL(+) group
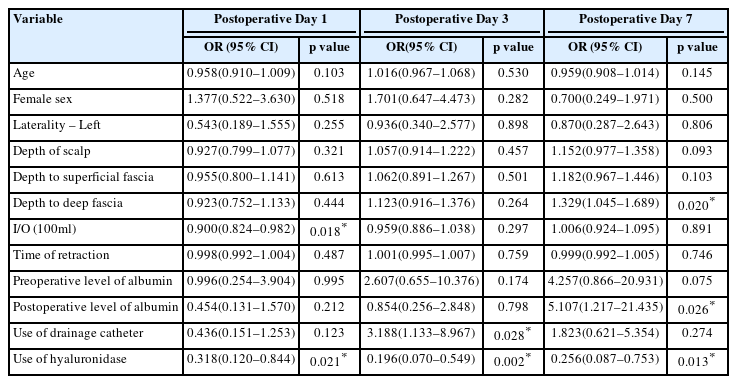
On postoperative day 1, intraoperative I/O (OR 0.900; 95% CI 0.824–0.982; p=0.018) and the use of hyaluronidase (OR 0.318; 95% CI 0.120–0.844; p=0.021) were significantly correlated with reduction of the periorbital edema with univariate ordinal logistic regression analysis. On postoperative day 3, the use of hyaluronidase (OR 0.196; 95% CI 0.070–0.549; p=0.002) significantly decreased the periorbital edema and the use of a drainage catheter (OR 3.188; 95% CI 1.133–8.967; p=0.028) significantly increased the periorbital edema. On postoperative day 7, the use of hyaluronidase (OR 0.256; 95% CI 0.087-0.753; p=0.013) significantly decreased the periorbital edema and the depth between bone and deep fascia of temporalis muscle (OR 1.329; 95% CI 1.045–1.689; p=0.020) and postoperative level of serum albumin (OR 5.107; 95% CI 1.217–21.435; p=0.026) significantly increased the periorbital edema with univariate ordinal logistic regression analysis (Table 2).
With multivariate ordinal logistic regression analysis including parameters of p-value 0.05, the use of hyaluronidase constantly showed a significant correlation with the reduction of periorbital edema on postoperative day 1 (OR 0.337; 95% CI 0.125–0.906; p=0.031), 3 (OR 0.230; 95% CI 0.081–0.655; p=0.006), and 7 (OR 0.160; 95% CI 0.046–0.553; p=0.004).
Postoperative pain based on NPIS
The postoperative pain showed a decline over time and the slope of the decline was steeper in HYAL(+) group (estimated slope=–0.505; p<0.0001) than in HYAL(-) group (estimated slope=–0.339) with linear mixed model (Fig. 3). Although the slope of the decline in postoperative pain was not significantly different between two groups, it showed a trend of difference (p=0.096).
DISCUSSION
Hyaluronic acid is a non-sulfated glycosaminoglycan and is the predominant part of the extracellular matrix of the skin11). Hyaluronidase is a soluble enzyme that degrades hyaluronic acid by hydrolyzation12) and was recognized as promoting the diffusion and dispersion of fluids and wound healing8). Based on these properties, hyaluronidase has been revealed to enhance the diffusion of the drug so that the efficacy of local anesthetics would be increased with the combination13,14). Additionally, hyaluronidase might accelerate wound healing process8,15). In Korea, hyaluronidase of bovine is commercially available and is permitted to be used in subcutaneous infusion, local anesthesia, extravasation, and hematoma.
The use of hyaluronidase in edematous conditions would be reasonable based on the property, but as we know, there was only one study of hyaluronidase injection for the treatment of eyelid edema5). Prior to the evaluation of periorbital edema, we made a grading system of periorbital edema based on the vertical palpebral aperture of the contralateral eye. It might be similar to the Modified Surgeon Periorbital Rating of Edema and Ecchymosis (SPREE) questionnaire, but the SPREE did not classify the edema quantitatively and also focused on the ecchymosis16).
Although the surgery for unruptured intracranial aneurysms has been developed less invasively, the periorbital edema occurred as ever6). There have been efforts to reduce periorbital edema, including steroid injection and cryotherapy10,17). In the current study, we used hyaluronidase to reduce the periorbital edema after surgery for unruptured intracranial aneurysms. Hyaluronidase reduced the periorbital edema effectively and constantly without any related complications during the postoperative period.
Otherwise, on postoperative day 3, the use of a drainage catheter was correlated with increase in periorbital edema. Generally, the drainage catheter would be removed on postoperative day 1 or 2. The periorbital edema might be worsened just after the removal of drainage catheter because of fluid collection. And on postoperative day 7, the depth between bone and deep fascia of temporalis muscle was significantly correlated with the periorbital edema. The periorbital edema timely decreased, so that the thickness of temporalis muscle itself might reflect the degree of the periorbital edema on postoperative day 7.
The main limitation of the study is the retrospective design and selection bias derived from the low inclusion rate (60 of 280 patients), because only patients with postoperative photographs were included to the study. Second, the grading system does not quantitatively reflect the degree of periorbital edema. To minimize subjective judgement of periorbital edema, we made a grading system of periorbital edema based on the vertical palpebral aperture of the contralateral eye.
CONCLUSION
In the current study, the use of hyaluronidase might be helpful to reduce the periorbital edema effectively after surgery for unruptured intracranial aneurysms during the postoperative period without any related complications. Furthermore, a randomized study of a larger population to identify the effect of hyaluronidase on the reduction of periorbital edema might be required.
Notes
Ethics statement
The authors had received approval from the Institutional Review Board. Informed Consent was waived, based on the retrospective design of the current study.
Author contributions
Conceptualization, Data curation, Formal analysis: JJK. Methodology: CHJ. Writing – original draft: SY. Writing – review & editing: CHJ, JJK
Conflict of interest
There is no conflict of interest to disclose.
Funding
None.
Data availability
None.
Acknowledgements
The authors thank Medical Illustration & Design, part of the Medical Research Support Services of Yonsei University College of Medicine, for all artistic support related to this work.